COVID-19
LSTM is playing a unique role in the response to COVID-19, covering all of the translational research cycle. From the beginning of the outbreak It has partnered with industry, academic institutions and organisations aimed to have the best possible impact on public health in the UK and overseas.
A lot of LSTM’s research is conducted via its Centre for Drugs and Diagnostics (CDD). This Centre comprises an experienced multi-disciplinary group of experts working together researching, developing and validating drugs and diagnostics in response to the COVID-19 pandemic.
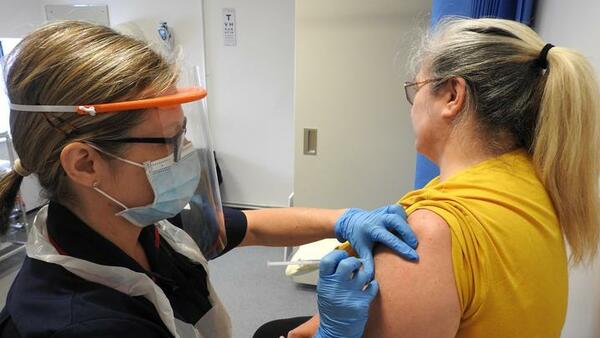
Research into vaccines is the only way to find out which vaccines will work. LSTM plays a key role in the various COVID-19 vaccine research programmes that are ongoing, most notably establishing LSTM as a Phase III trial site for the Oxford Vaccine Trial.
LSTM also partners with multiple other institutions and organisations to provide evidence based advisory services to government departments and other interested parties.
COVID-19 research activities at LSTM
Well Travelled Clinics and COVID-19

Since the early days of the pandemic, staff at LSTM’s Well Travelled Clinics (WTC) have played a significant role in supporting LSTM and local NHS and PHE health care teams in their response to COVID-19.
Improving Health Systems: Adjusting to COVID-19
LSTM researchers, led by Professors Sally Theobald, Miriam Taegtmeyer, Imelda Bates and Dr. Laura Dean and partner organisations (University of Liverpool & Ministry of Health, Liberia, and Liverpool Hospitals NHS Foundation Trust) are currently looking into how health systems in Liverpool and Liberia have been adjusting to the ongoing COVID-19 outbreak.
COVID-19 Latest news
-
 From: LSTMStudy finds severe disease from Omicron B.1.1529 SARS-CoV-2 infections are uncommon in under-vaccinated Malawian communityNews article19 Jan 2023
From: LSTMStudy finds severe disease from Omicron B.1.1529 SARS-CoV-2 infections are uncommon in under-vaccinated Malawian communityNews article19 Jan 2023 -
 From: LSTMLSTM and Partners Win at the North West Coast Research and Innovation Awards 2022News article24 Jun 2022
From: LSTMLSTM and Partners Win at the North West Coast Research and Innovation Awards 2022News article24 Jun 2022 -
 From: LSTMGlobal insights support programme to boost vaccine uptake within Liverpool communitiesNews article26 Apr 2022
From: LSTMGlobal insights support programme to boost vaccine uptake within Liverpool communitiesNews article26 Apr 2022 -
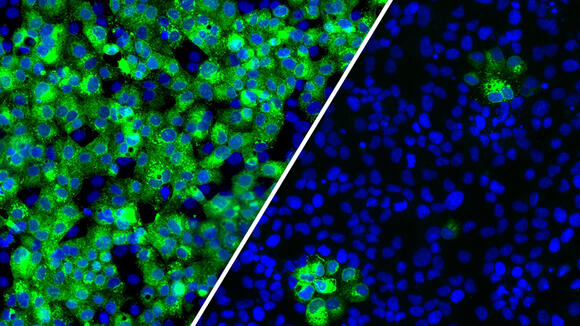 From: LSTMLSTM researchers find targeting glycosylation blocks SARS-CoV-2 infectionNews article15 Feb 2022
From: LSTMLSTM researchers find targeting glycosylation blocks SARS-CoV-2 infectionNews article15 Feb 2022 -
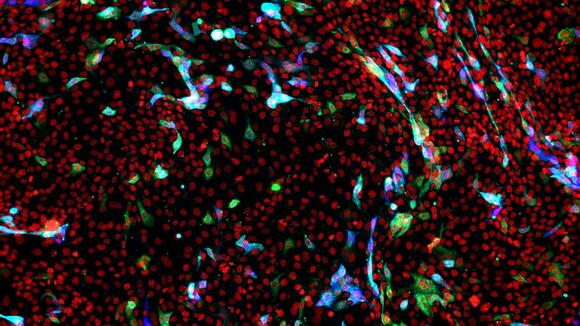 From: LSTMUsing live imaging microscopy at LSTM to film COVID-19 infecting cellsNews article10 Nov 2021
From: LSTMUsing live imaging microscopy at LSTM to film COVID-19 infecting cellsNews article10 Nov 2021 -
 From: LSTMAGILE Covid-19 drug testing platform opens new trial in South AfricaNews article7 Oct 2021
From: LSTMAGILE Covid-19 drug testing platform opens new trial in South AfricaNews article7 Oct 2021 -
 From: LSTMIn conversation: DTMH student Dr Lia Lopez and LSTM's Dr Tom WingfieldBlog3 Sep 2021
From: LSTMIn conversation: DTMH student Dr Lia Lopez and LSTM's Dr Tom WingfieldBlog3 Sep 2021 -
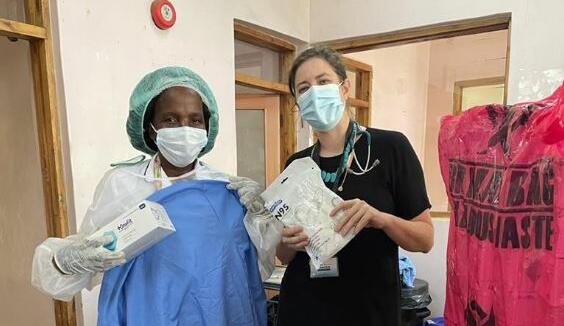
 From: LSTMBump it Forward: Donations arrive in Freetown Sierra LeoneNews article20 Aug 2021
From: LSTMBump it Forward: Donations arrive in Freetown Sierra LeoneNews article20 Aug 2021 -
 From: LSTMIvermectin treatment in humans for COVID-19News article28 Jul 2021
From: LSTMIvermectin treatment in humans for COVID-19News article28 Jul 2021 -
 From: LSTMLSTM contributes to India specific guidelines for the treatment and management of COVID-19News article22 Jun 2021
From: LSTMLSTM contributes to India specific guidelines for the treatment and management of COVID-19News article22 Jun 2021 -
 From: LSTMLSTM and MLW Programme study in Nature Communications: exploring diagnosis and treatment of COVID-19 in Sub-Saharan AfricaNews article11 Jun 2021
From: LSTMLSTM and MLW Programme study in Nature Communications: exploring diagnosis and treatment of COVID-19 in Sub-Saharan AfricaNews article11 Jun 2021 -
 From: LSTM#Quality Assurance for PPEsBlog19 May 2021
From: LSTM#Quality Assurance for PPEsBlog19 May 2021 -
 From: LSTMFinal K4D COVID-19 Health Evidence Summary publishedNews article11 May 2021
From: LSTMFinal K4D COVID-19 Health Evidence Summary publishedNews article11 May 2021 -
 From: LSTMFirst patient dosed in latest stage of AGILE COVID-19 drug trialNews article20 Apr 2021
From: LSTMFirst patient dosed in latest stage of AGILE COVID-19 drug trialNews article20 Apr 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia14 Apr 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia14 Apr 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Helen HillMedia14 Apr 2021
From: LSTMBBC Radio Merseyside with LSTM's Dr Helen HillMedia14 Apr 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia13 Apr 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia13 Apr 2021 -
From: LSTMJoint Open Editorial LSTM, IDS & LSHTM: The government's aid funding cuts are a wrecking ball to global health securityNews article1 Apr 2021
-
 From: LSTMBump it Forward campaign makes further PPE donations in 6 African countriesNews article29 Mar 2021
From: LSTMBump it Forward campaign makes further PPE donations in 6 African countriesNews article29 Mar 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia22 Mar 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia22 Mar 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia22 Mar 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia22 Mar 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia11 Mar 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia11 Mar 2021 -
 From: LSTMCoffee roastery donates some of its profits to support LSTM’s ‘Bump It Forward' campaignNews article25 Feb 2021
From: LSTMCoffee roastery donates some of its profits to support LSTM’s ‘Bump It Forward' campaignNews article25 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia22 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia22 Feb 2021 -
 From: LSTMResults of first COVID patient survey publishedNews article17 Feb 2021
From: LSTMResults of first COVID patient survey publishedNews article17 Feb 2021 -
From: LSTMBump It Forward campaign donations start to make an impactNews article17 Feb 2021
-
 From: LSTMCOVID-19 drug testing platform AGILE receives government funding to fast-track clinical trialsNews article13 Feb 2021
From: LSTMCOVID-19 drug testing platform AGILE receives government funding to fast-track clinical trialsNews article13 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Rachel ByrneMedia11 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Rachel ByrneMedia11 Feb 2021 -
 From: LSTMNew Cochrane Review shows chloroquine doesn’t help people with COVID-19News article11 Feb 2021
From: LSTMNew Cochrane Review shows chloroquine doesn’t help people with COVID-19News article11 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom EdwardsMedia11 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom EdwardsMedia11 Feb 2021 -
 From: LSTMTransmission risk of COVID-19 from sports equipmentNews article10 Feb 2021
From: LSTMTransmission risk of COVID-19 from sports equipmentNews article10 Feb 2021 -
 From: LSTMA community conversation about the COVID 19 VaccineEvent10 Feb 2021
From: LSTMA community conversation about the COVID 19 VaccineEvent10 Feb 2021 -
 From: LSTM£65,000 fundraising milestone reached in ‘Bump It Forward’ CampaignNews article8 Feb 2021
From: LSTM£65,000 fundraising milestone reached in ‘Bump It Forward’ CampaignNews article8 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Kelly DaviesMedia5 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Kelly DaviesMedia5 Feb 2021 -
 From: LSTMLiverpool's role in the vaccine - A podcastBlog4 Feb 2021
From: LSTMLiverpool's role in the vaccine - A podcastBlog4 Feb 2021 -
From: LSTMBBC Radio Merseyside with LSTM's Dr Helen HillMedia4 Feb 2021
-
 From: LSTMVolunteer for the COM COV Clinical TrialNews article4 Feb 2021
From: LSTMVolunteer for the COM COV Clinical TrialNews article4 Feb 2021 -
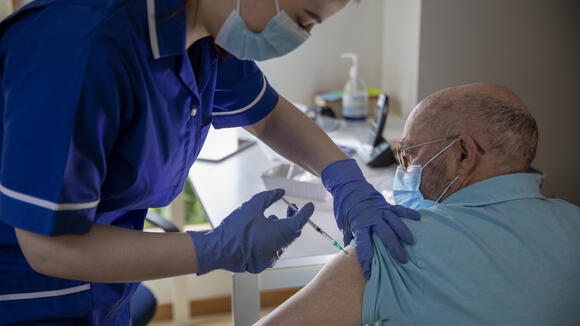 From: LSTMWorld's first COVID-19 vaccine alternating dose study launches in LiverpoolNews article3 Feb 2021
From: LSTMWorld's first COVID-19 vaccine alternating dose study launches in LiverpoolNews article3 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia3 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia3 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia2 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia2 Feb 2021 -
From: LSTMProf. Melita Gordon and Dr Fumbani Limani talk about the Bump it Forward campaign on BBC Radio MerseysideMedia1 Feb 2021
-
 From: LSTMBBC Radio Merseyside with LSTM's Dr Jamie RylanceMedia28 Jan 2021
From: LSTMBBC Radio Merseyside with LSTM's Dr Jamie RylanceMedia28 Jan 2021 -
 From: LSTMLSTM launches 125th anniversary Webinar SeriesNews article27 Jan 2021
From: LSTMLSTM launches 125th anniversary Webinar SeriesNews article27 Jan 2021 -
 From: LSTMLSTM receives £3 million from Research EnglandNews article25 Jan 2021
From: LSTMLSTM receives £3 million from Research EnglandNews article25 Jan 2021 -
 From: LSTMLSTM launches Bump It Forward campaignNews article21 Jan 2021
From: LSTMLSTM launches Bump It Forward campaignNews article21 Jan 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia18 Jan 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia18 Jan 2021 -
 From: LSTMExcalibur rapid SARS-COV-2 antigen screening test, validated by LSTM, granted MHRA approvalNews article13 Jan 2021
From: LSTMExcalibur rapid SARS-COV-2 antigen screening test, validated by LSTM, granted MHRA approvalNews article13 Jan 2021 -
 From: LSTMTwo new therapies to be tested in ground-breaking COVID-19 clinical trialNews article12 Jan 2021
From: LSTMTwo new therapies to be tested in ground-breaking COVID-19 clinical trialNews article12 Jan 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia11 Jan 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia11 Jan 2021 -
 From: LSTMMologic Receives CE Mark Approval for Professional-Use COVID-19 Rapid Antigen TestNews article24 Dec 2020
From: LSTMMologic Receives CE Mark Approval for Professional-Use COVID-19 Rapid Antigen TestNews article24 Dec 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia17 Dec 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia17 Dec 2020 -
 From: LSTMNew spray, co-developed with the British Army and tested by LSTM, kills coronavirus in under 60 secondsNews article17 Dec 2020
From: LSTMNew spray, co-developed with the British Army and tested by LSTM, kills coronavirus in under 60 secondsNews article17 Dec 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia10 Dec 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia10 Dec 2020 -
From: LSTMOxford Covid vaccine 'safe and effective', Lancet study showsNews article9 Dec 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia8 Dec 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia8 Dec 2020 -
 From: LSTMEvidence of exposure to SARS-CoV-2 in cats and dogs from households in ItalyNews article4 Dec 2020
From: LSTMEvidence of exposure to SARS-CoV-2 in cats and dogs from households in ItalyNews article4 Dec 2020 -
 From: LSTMThe interchange between climate change, COVID-19 and responseSeminars & lectures3 Dec 2020
From: LSTMThe interchange between climate change, COVID-19 and responseSeminars & lectures3 Dec 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia3 Dec 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia3 Dec 2020 -
 From: LSTMLSTM’s Professor Miriam Taegtmeyer and Dr Tom Wingfield in The Conversation: ‘We will not forget our colleagues who have died’News article2 Dec 2020
From: LSTMLSTM’s Professor Miriam Taegtmeyer and Dr Tom Wingfield in The Conversation: ‘We will not forget our colleagues who have died’News article2 Dec 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia30 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia30 Nov 2020 -
 From: LSTMLSTM Annual Report and Financial Statements 2019-2020News article26 Nov 2020
From: LSTMLSTM Annual Report and Financial Statements 2019-2020News article26 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia26 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia26 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia20 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia20 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia19 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia19 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia12 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia12 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Sara BeggMedia11 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Sara BeggMedia11 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia10 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia10 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia5 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia5 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia4 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia4 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia29 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia29 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia28 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia28 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia26 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia26 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia22 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia22 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia19 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia19 Oct 2020 -
 From: LSTMWorld Mental Health Day 2020: one LSTM student’s research on mental health amongst health workers in Sierra LeoneNews article9 Oct 2020
From: LSTMWorld Mental Health Day 2020: one LSTM student’s research on mental health amongst health workers in Sierra LeoneNews article9 Oct 2020 -
 From: LSTMWorld Mental Health Day 2020. Protecting the mental health of the global health workforce during COVID-19: Lessons learned from the Ebola outbreakNews article9 Oct 2020
From: LSTMWorld Mental Health Day 2020. Protecting the mental health of the global health workforce during COVID-19: Lessons learned from the Ebola outbreakNews article9 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia1 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia1 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia29 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia29 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia24 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia24 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia23 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia23 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia23 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia23 Sep 2020 -
 From: LSTMAGILE trial now recruiting patients for efficacy of COVID-19 treatmentsNews article18 Sep 2020
From: LSTMAGILE trial now recruiting patients for efficacy of COVID-19 treatmentsNews article18 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia17 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia17 Sep 2020 -
 From: LSTMLSTM researchers find an easy alternative to nasal and throat swabbing for SARS-CoV-2 DiagnosisNews article14 Sep 2020
From: LSTMLSTM researchers find an easy alternative to nasal and throat swabbing for SARS-CoV-2 DiagnosisNews article14 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia14 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia14 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia11 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia11 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia9 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia9 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia8 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia8 Sep 2020 -
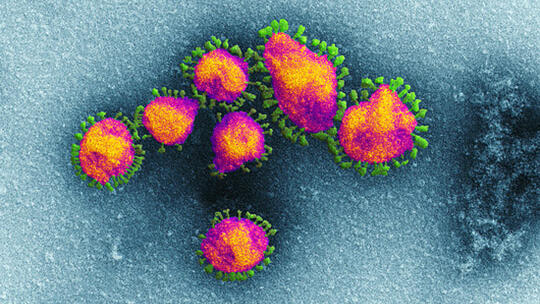 From: LSTMPaul Garner on long haul covid-19—Don’t try to dominate this virus, accommodate itBlog4 Sep 2020
From: LSTMPaul Garner on long haul covid-19—Don’t try to dominate this virus, accommodate itBlog4 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia3 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia3 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia2 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia2 Sep 2020 -
 From: LSTMBMJ Webinar: 'Long COVID: diagnosis, management, prognosis'Event3 Sep 2020
From: LSTMBMJ Webinar: 'Long COVID: diagnosis, management, prognosis'Event3 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia27 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia27 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Senior Clinical Lecturer Dr Tom WingfieldMedia24 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Senior Clinical Lecturer Dr Tom WingfieldMedia24 Aug 2020 -
From: LSTMWould you like to volunteer to take part in COVID -19 Vaccine Research?News article20 Aug 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia20 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia20 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia18 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia18 Aug 2020 -
 From: LSTMCall for volunteers: COVID-LIV Virology StudyNews article17 Aug 2020
From: LSTMCall for volunteers: COVID-LIV Virology StudyNews article17 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia17 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia17 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia13 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia13 Aug 2020 -
From: LSTMCOVID-19 campaign to reach millions in 9 African countriesNews article11 Aug 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Dr Aitor Casas-SanchezMedia11 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Aitor Casas-SanchezMedia11 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Rachel ByrneMedia4 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Rachel ByrneMedia4 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia30 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia30 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Ian PattersonMedia28 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Ian PattersonMedia28 Jul 2020 -
 From: LSTMEvidence of exposure to SARS-CoV-2 in cats and dogs from households in ItalyNews article27 Jul 2020
From: LSTMEvidence of exposure to SARS-CoV-2 in cats and dogs from households in ItalyNews article27 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia23 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia23 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia21 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia21 Jul 2020 -
 From: LSTMSaliva offers a non-invasive alternative to upper respiratory swabs for COVID-19 diagnosisNews article17 Jul 2020
From: LSTMSaliva offers a non-invasive alternative to upper respiratory swabs for COVID-19 diagnosisNews article17 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia17 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia17 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia16 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia16 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia14 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia14 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia13 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia13 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia9 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia9 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia8 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia8 Jul 2020 -
 From: LSTMLSTM's Prof. Paul Garner on BBC Radio 5 LiveMedia6 Jul 2020
From: LSTMLSTM's Prof. Paul Garner on BBC Radio 5 LiveMedia6 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Sarah Lewis NewtonMedia6 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Sarah Lewis NewtonMedia6 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia2 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia2 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia1 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia1 Jul 2020 -
 From: LSTMMLW Programme opens oxygen concentration plant in Blantyre, MalawiNews article29 Jun 2020
From: LSTMMLW Programme opens oxygen concentration plant in Blantyre, MalawiNews article29 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Janet HemingwayMedia29 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Janet HemingwayMedia29 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Nurse Kate BradfieldMedia26 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Nurse Kate BradfieldMedia26 Jun 2020 -
From: LSTMLSTM-led consortium receives £18.6m from UKRINews article26 Jun 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia25 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia25 Jun 2020 -
 From: LSTMPaul Garner: Covid-19 at 14 weeks—phantom speed cameras, unknown limits, and harsh penaltiesBlog23 Jun 2020
From: LSTMPaul Garner: Covid-19 at 14 weeks—phantom speed cameras, unknown limits, and harsh penaltiesBlog23 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Ian PattersonMedia23 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Ian PattersonMedia23 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Laura JeffreysMedia19 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Laura JeffreysMedia19 Jun 2020 -
 From: LSTMLSTM's third virtual symposium on Global Health Research & Vaccination: Private sector partnership, research and accelerating the development of new toolsNews article18 Jun 2020
From: LSTMLSTM's third virtual symposium on Global Health Research & Vaccination: Private sector partnership, research and accelerating the development of new toolsNews article18 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia17 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia17 Jun 2020 -
 From: LSTMLMICs will face 'extreme strain' on health systems due to COVID-19 despite younger populationsNews article12 Jun 2020
From: LSTMLMICs will face 'extreme strain' on health systems due to COVID-19 despite younger populationsNews article12 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Carla Solórzano-GonzalezMedia12 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Carla Solórzano-GonzalezMedia12 Jun 2020 -
 From: LSTMUnique Urwerk watch raises over £33,000 for LSTM's COVID-19 researchNews article12 Jun 2020
From: LSTMUnique Urwerk watch raises over £33,000 for LSTM's COVID-19 researchNews article12 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia11 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia11 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia9 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia9 Jun 2020 -
 From: LSTMMore than 250 Merseyside volunteers vaccinated in COVID-19 Oxford vaccine trialNews article8 Jun 2020
From: LSTMMore than 250 Merseyside volunteers vaccinated in COVID-19 Oxford vaccine trialNews article8 Jun 2020 -
 From: LSTMCOVID-19 antibody test validated by LSTM researchers ready for manufacture and distributionNews article4 Jun 2020
From: LSTMCOVID-19 antibody test validated by LSTM researchers ready for manufacture and distributionNews article4 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia4 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia4 Jun 2020 -
 From: LSTMScreening of licensed drugs to potentially treat COVID-19Blog3 Jun 2020
From: LSTMScreening of licensed drugs to potentially treat COVID-19Blog3 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Daniela FerreiraMedia3 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Daniela FerreiraMedia3 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Janet HemingwayMedia1 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Janet HemingwayMedia1 Jun 2020 -
 From: LSTMCOVID-19 Oxford Vaccine Trial: LSTM is currently recruiting volunteersNews article28 May 2020
From: LSTMCOVID-19 Oxford Vaccine Trial: LSTM is currently recruiting volunteersNews article28 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia28 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia28 May 2020 -
From: LSTM'Access, equity and delivery': LSTM’s second virtual symposium on Global Health Research and VaccinationNews article28 May 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia27 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia27 May 2020 -
 From: LSTMLSTM named as a site for Phase III of the Oxford COVID-19 vaccine human trialsNews article22 May 2020
From: LSTMLSTM named as a site for Phase III of the Oxford COVID-19 vaccine human trialsNews article22 May 2020 -
 From: LSTMLSTM's Dr Andrea Collins on BBC Radio MerseysideMedia22 May 2020
From: LSTMLSTM's Dr Andrea Collins on BBC Radio MerseysideMedia22 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia21 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia21 May 2020 -
 From: LSTMLSTM's Dr Lee Haines on BBC Radio MerseysideMedia20 May 2020
From: LSTMLSTM's Dr Lee Haines on BBC Radio MerseysideMedia20 May 2020 -
 From: LSTMCovid19 and fatigue - a game of snakes and laddersBlog20 May 2020
From: LSTMCovid19 and fatigue - a game of snakes and laddersBlog20 May 2020 -
 From: LSTMLSTM's Dr Shaun Pennington on BBC Radio MerseysideMedia19 May 2020
From: LSTMLSTM's Dr Shaun Pennington on BBC Radio MerseysideMedia19 May 2020 -
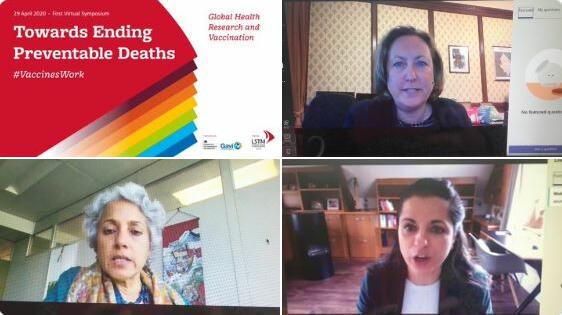
 From: LSTMTowards ending preventable deaths: Global health research and vaccinations in the context of emerging pandemics – answering your questions from our first global symposiumBlog18 May 2020
From: LSTMTowards ending preventable deaths: Global health research and vaccinations in the context of emerging pandemics – answering your questions from our first global symposiumBlog18 May 2020 -
From: LSTMPaul Garner: ‘The virus is causing lots of immunological changes in the body, lots of pathology that we don’t yet understand.’Blog15 May 2020
-
 From: LSTMLSTM led study: While adapting to COVID-19 can Liverpool’s health system learn from the West African Ebola outbreak?News article15 May 2020
From: LSTMLSTM led study: While adapting to COVID-19 can Liverpool’s health system learn from the West African Ebola outbreak?News article15 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia15 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia15 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia14 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia14 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Adam RobertsMedia13 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Adam RobertsMedia13 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia7 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia7 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Samantha DonnellanMedia6 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Samantha DonnellanMedia6 May 2020 -
 From: LSTMPaul Garner: For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustionBlog5 May 2020
From: LSTMPaul Garner: For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustionBlog5 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Imelda BatesMedia4 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Imelda BatesMedia4 May 2020 -
 From: LSTMVirtual Race to Malawi ChallengeNews article1 May 2020
From: LSTMVirtual Race to Malawi ChallengeNews article1 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia30 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia30 Apr 2020 -
From: LSTMLSTM begins series of virtual vaccine research symposia with global guests and new funding pledgeNews article30 Apr 2020
-
 From: LSTMGlobal Health research & vaccination, in the context of emerging pandemics towards ending preventable deathsSeminars & lectures29 Apr 2020
From: LSTMGlobal Health research & vaccination, in the context of emerging pandemics towards ending preventable deathsSeminars & lectures29 Apr 2020 -
 From: LSTMMy letter to LiverpoolBlog29 Apr 2020
From: LSTMMy letter to LiverpoolBlog29 Apr 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Sean TomlinsonMedia28 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Sean TomlinsonMedia28 Apr 2020 -
 From: LSTMLSTM & World Immunization Week 2020News article27 Apr 2020
From: LSTMLSTM & World Immunization Week 2020News article27 Apr 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Andrea CollinsMedia24 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Andrea CollinsMedia24 Apr 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia23 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia23 Apr 2020 -
 From: LSTMGrowing support for LSTM’s COVID-19 Response and Resilience Fund in MalawiNews article22 Apr 2020
From: LSTMGrowing support for LSTM’s COVID-19 Response and Resilience Fund in MalawiNews article22 Apr 2020 -
 From: LSTMAn open letter from colleagues at the Malawi-Liverpool-Wellcome Trust Clinical Research ProgrammeNews article21 Apr 2020
From: LSTMAn open letter from colleagues at the Malawi-Liverpool-Wellcome Trust Clinical Research ProgrammeNews article21 Apr 2020 -
 From: LSTMLSTM’s 3D printers reallocated to print face shields for NHS frontline staffNews article21 Apr 2020
From: LSTMLSTM’s 3D printers reallocated to print face shields for NHS frontline staffNews article21 Apr 2020 -
 From: LSTMMeet the team of our CL3 containment laboratories conducting some of LSTM's Covid-19 researchBlog20 Apr 2020
From: LSTMMeet the team of our CL3 containment laboratories conducting some of LSTM's Covid-19 researchBlog20 Apr 2020 -
 From: LSTMCovid-19 & Maternal Health: Are we facing a new normal?Event16 Apr 2020
From: LSTMCovid-19 & Maternal Health: Are we facing a new normal?Event16 Apr 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Senior Clinical Lecturer Dr Tom WingfieldMedia14 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Senior Clinical Lecturer Dr Tom WingfieldMedia14 Apr 2020 -
From: LSTMLSTM and Pfizer Inc. announce two new studies on the interaction between S. pneumoniae and SARS-CoV-2News article9 Apr 2020
-
 From: LSTM“To an unpredictable future!” - in times of DTM&H - Corona editionBlog8 Apr 2020
From: LSTM“To an unpredictable future!” - in times of DTM&H - Corona editionBlog8 Apr 2020 -
 From: LSTMBMJ editorial: LSTM clinicians call for rapid near-patient testing for COVID-19News article8 Apr 2020
From: LSTMBMJ editorial: LSTM clinicians call for rapid near-patient testing for COVID-19News article8 Apr 2020 -
 From: LSTMLSTM researchers look at the impact of COVID-19 on healthcare workersNews article7 Apr 2020
From: LSTMLSTM researchers look at the impact of COVID-19 on healthcare workersNews article7 Apr 2020 -
 From: LSTMBBC Radio Merseyside's SciFri with Rachel Byrne MRC/DTP PhD candidateMedia3 Apr 2020
From: LSTMBBC Radio Merseyside's SciFri with Rachel Byrne MRC/DTP PhD candidateMedia3 Apr 2020 -
 From: LSTMLSTM key partner in publishing K4D COVID-19 Health Evidence SummariesNews article3 Apr 2020
From: LSTMLSTM key partner in publishing K4D COVID-19 Health Evidence SummariesNews article3 Apr 2020 -
 From: LSTMLSTM joins global coalition to accelerate research on the prevention and treatment of COVID-19 in low- and middle-income countriesNews article2 Apr 2020
From: LSTMLSTM joins global coalition to accelerate research on the prevention and treatment of COVID-19 in low- and middle-income countriesNews article2 Apr 2020 -
 From: LSTMCOVID-19 and disability: virtual Q&AEvent6 Apr 2020
From: LSTMCOVID-19 and disability: virtual Q&AEvent6 Apr 2020 -
 From: LSTMAntibiotic resistance could lead to more COVID-19 deathsBlog2 Apr 2020
From: LSTMAntibiotic resistance could lead to more COVID-19 deathsBlog2 Apr 2020 -
 From: LSTMLSTM launches fundraising appeal to support COVID-19 research and response in MalawiNews article1 Apr 2020
From: LSTMLSTM launches fundraising appeal to support COVID-19 research and response in MalawiNews article1 Apr 2020 -
 From: LSTMPandemic Preparedness PurseBlog1 Apr 2020
From: LSTMPandemic Preparedness PurseBlog1 Apr 2020 -
 From: LSTMLSTM's Respiratory Clinical Research Group conducts key COVID-19 research in patients & healthcare workersNews article31 Mar 2020
From: LSTMLSTM's Respiratory Clinical Research Group conducts key COVID-19 research in patients & healthcare workersNews article31 Mar 2020 -
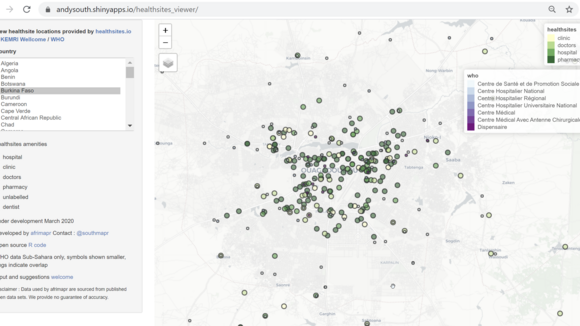 From: LSTMOpen data and software to support the COVID-19 response in AfricaNews article30 Mar 2020
From: LSTMOpen data and software to support the COVID-19 response in AfricaNews article30 Mar 2020 -
 From: LSTMLSTM begins the validation process for the Mologic COVID-19 point-of-need diagnostic testNews article27 Mar 2020
From: LSTMLSTM begins the validation process for the Mologic COVID-19 point-of-need diagnostic testNews article27 Mar 2020 -
 From: LSTMBlog: Don’t get complacent AMR; we’ll be backNews article25 Mar 2020
From: LSTMBlog: Don’t get complacent AMR; we’ll be backNews article25 Mar 2020 -
From: LSTMDon’t get complacent AMR; we’ll be backBlog25 Mar 2020
-
 From: LSTM“Antibodies will make a significant contribution to design effective control strategies for COVID-19”News article24 Mar 2020
From: LSTM“Antibodies will make a significant contribution to design effective control strategies for COVID-19”News article24 Mar 2020 -
 From: LSTMA Tale of Two Pandemics: a plea on World TB DayNews article24 Mar 2020
From: LSTMA Tale of Two Pandemics: a plea on World TB DayNews article24 Mar 2020 -
 From: LSTMCOVID-19: LSTM's Dr Tom Wingfield answers questions from LSTM's overseas partnersNews article20 Mar 2020
From: LSTMCOVID-19: LSTM's Dr Tom Wingfield answers questions from LSTM's overseas partnersNews article20 Mar 2020 -
 From: LSTMLSTM researchers work on new diagnostics for COVID-19News article12 Mar 2020
From: LSTMLSTM researchers work on new diagnostics for COVID-19News article12 Mar 2020 -
 From: LSTMBMJ Best Practice monograph: COVID-19News article20 Feb 2020
From: LSTMBMJ Best Practice monograph: COVID-19News article20 Feb 2020
-
 From: LSTMStudy finds severe disease from Omicron B.1.1529 SARS-CoV-2 infections are uncommon in under-vaccinated Malawian communityNews article19 Jan 2023
From: LSTMStudy finds severe disease from Omicron B.1.1529 SARS-CoV-2 infections are uncommon in under-vaccinated Malawian communityNews article19 Jan 2023 -
 From: LSTMLSTM and Partners Win at the North West Coast Research and Innovation Awards 2022News article24 Jun 2022
From: LSTMLSTM and Partners Win at the North West Coast Research and Innovation Awards 2022News article24 Jun 2022 -
 From: LSTMGlobal insights support programme to boost vaccine uptake within Liverpool communitiesNews article26 Apr 2022
From: LSTMGlobal insights support programme to boost vaccine uptake within Liverpool communitiesNews article26 Apr 2022 -
 From: LSTMLSTM researchers find targeting glycosylation blocks SARS-CoV-2 infectionNews article15 Feb 2022
From: LSTMLSTM researchers find targeting glycosylation blocks SARS-CoV-2 infectionNews article15 Feb 2022 -
 From: LSTMUsing live imaging microscopy at LSTM to film COVID-19 infecting cellsNews article10 Nov 2021
From: LSTMUsing live imaging microscopy at LSTM to film COVID-19 infecting cellsNews article10 Nov 2021 -
 From: LSTMAGILE Covid-19 drug testing platform opens new trial in South AfricaNews article7 Oct 2021
From: LSTMAGILE Covid-19 drug testing platform opens new trial in South AfricaNews article7 Oct 2021 -
 From: LSTMIn conversation: DTMH student Dr Lia Lopez and LSTM's Dr Tom WingfieldBlog3 Sep 2021
From: LSTMIn conversation: DTMH student Dr Lia Lopez and LSTM's Dr Tom WingfieldBlog3 Sep 2021 -
 From: LSTMBump it Forward: Donations arrive in Freetown Sierra LeoneNews article20 Aug 2021
From: LSTMBump it Forward: Donations arrive in Freetown Sierra LeoneNews article20 Aug 2021 -
 From: LSTMIvermectin treatment in humans for COVID-19News article28 Jul 2021
From: LSTMIvermectin treatment in humans for COVID-19News article28 Jul 2021 -
 From: LSTMLSTM contributes to India specific guidelines for the treatment and management of COVID-19News article22 Jun 2021
From: LSTMLSTM contributes to India specific guidelines for the treatment and management of COVID-19News article22 Jun 2021 -
 From: LSTMLSTM and MLW Programme study in Nature Communications: exploring diagnosis and treatment of COVID-19 in Sub-Saharan AfricaNews article11 Jun 2021
From: LSTMLSTM and MLW Programme study in Nature Communications: exploring diagnosis and treatment of COVID-19 in Sub-Saharan AfricaNews article11 Jun 2021 -
 From: LSTM#Quality Assurance for PPEsBlog19 May 2021
From: LSTM#Quality Assurance for PPEsBlog19 May 2021 -
 From: LSTMFinal K4D COVID-19 Health Evidence Summary publishedNews article11 May 2021
From: LSTMFinal K4D COVID-19 Health Evidence Summary publishedNews article11 May 2021 -
 From: LSTMFirst patient dosed in latest stage of AGILE COVID-19 drug trialNews article20 Apr 2021
From: LSTMFirst patient dosed in latest stage of AGILE COVID-19 drug trialNews article20 Apr 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia14 Apr 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia14 Apr 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Helen HillMedia14 Apr 2021
From: LSTMBBC Radio Merseyside with LSTM's Dr Helen HillMedia14 Apr 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia13 Apr 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia13 Apr 2021 -
From: LSTMJoint Open Editorial LSTM, IDS & LSHTM: The government's aid funding cuts are a wrecking ball to global health securityNews article1 Apr 2021
-
 From: LSTMBump it Forward campaign makes further PPE donations in 6 African countriesNews article29 Mar 2021
From: LSTMBump it Forward campaign makes further PPE donations in 6 African countriesNews article29 Mar 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia22 Mar 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia22 Mar 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia22 Mar 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia22 Mar 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia11 Mar 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia11 Mar 2021 -
 From: LSTMCoffee roastery donates some of its profits to support LSTM’s ‘Bump It Forward' campaignNews article25 Feb 2021
From: LSTMCoffee roastery donates some of its profits to support LSTM’s ‘Bump It Forward' campaignNews article25 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia22 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia22 Feb 2021 -
 From: LSTMResults of first COVID patient survey publishedNews article17 Feb 2021
From: LSTMResults of first COVID patient survey publishedNews article17 Feb 2021 -
From: LSTMBump It Forward campaign donations start to make an impactNews article17 Feb 2021
-
 From: LSTMCOVID-19 drug testing platform AGILE receives government funding to fast-track clinical trialsNews article13 Feb 2021
From: LSTMCOVID-19 drug testing platform AGILE receives government funding to fast-track clinical trialsNews article13 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Rachel ByrneMedia11 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Rachel ByrneMedia11 Feb 2021 -
 From: LSTMNew Cochrane Review shows chloroquine doesn’t help people with COVID-19News article11 Feb 2021
From: LSTMNew Cochrane Review shows chloroquine doesn’t help people with COVID-19News article11 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom EdwardsMedia11 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom EdwardsMedia11 Feb 2021 -
 From: LSTMTransmission risk of COVID-19 from sports equipmentNews article10 Feb 2021
From: LSTMTransmission risk of COVID-19 from sports equipmentNews article10 Feb 2021 -
 From: LSTMA community conversation about the COVID 19 VaccineEvent10 Feb 2021
From: LSTMA community conversation about the COVID 19 VaccineEvent10 Feb 2021 -
 From: LSTM£65,000 fundraising milestone reached in ‘Bump It Forward’ CampaignNews article8 Feb 2021
From: LSTM£65,000 fundraising milestone reached in ‘Bump It Forward’ CampaignNews article8 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Kelly DaviesMedia5 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Kelly DaviesMedia5 Feb 2021 -
 From: LSTMLiverpool's role in the vaccine - A podcastBlog4 Feb 2021
From: LSTMLiverpool's role in the vaccine - A podcastBlog4 Feb 2021 -
From: LSTMBBC Radio Merseyside with LSTM's Dr Helen HillMedia4 Feb 2021
-
 From: LSTMVolunteer for the COM COV Clinical TrialNews article4 Feb 2021
From: LSTMVolunteer for the COM COV Clinical TrialNews article4 Feb 2021 -
 From: LSTMWorld's first COVID-19 vaccine alternating dose study launches in LiverpoolNews article3 Feb 2021
From: LSTMWorld's first COVID-19 vaccine alternating dose study launches in LiverpoolNews article3 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia3 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia3 Feb 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia2 Feb 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia2 Feb 2021 -
From: LSTMProf. Melita Gordon and Dr Fumbani Limani talk about the Bump it Forward campaign on BBC Radio MerseysideMedia1 Feb 2021
-
 From: LSTMBBC Radio Merseyside with LSTM's Dr Jamie RylanceMedia28 Jan 2021
From: LSTMBBC Radio Merseyside with LSTM's Dr Jamie RylanceMedia28 Jan 2021 -
 From: LSTMLSTM launches 125th anniversary Webinar SeriesNews article27 Jan 2021
From: LSTMLSTM launches 125th anniversary Webinar SeriesNews article27 Jan 2021 -
 From: LSTMLSTM receives £3 million from Research EnglandNews article25 Jan 2021
From: LSTMLSTM receives £3 million from Research EnglandNews article25 Jan 2021 -
 From: LSTMLSTM launches Bump It Forward campaignNews article21 Jan 2021
From: LSTMLSTM launches Bump It Forward campaignNews article21 Jan 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia18 Jan 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia18 Jan 2021 -
 From: LSTMExcalibur rapid SARS-COV-2 antigen screening test, validated by LSTM, granted MHRA approvalNews article13 Jan 2021
From: LSTMExcalibur rapid SARS-COV-2 antigen screening test, validated by LSTM, granted MHRA approvalNews article13 Jan 2021 -
 From: LSTMTwo new therapies to be tested in ground-breaking COVID-19 clinical trialNews article12 Jan 2021
From: LSTMTwo new therapies to be tested in ground-breaking COVID-19 clinical trialNews article12 Jan 2021 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia11 Jan 2021
From: LSTMBBC Radio Merseyside with LSTM's Professor Nick BeechingMedia11 Jan 2021 -
 From: LSTMMologic Receives CE Mark Approval for Professional-Use COVID-19 Rapid Antigen TestNews article24 Dec 2020
From: LSTMMologic Receives CE Mark Approval for Professional-Use COVID-19 Rapid Antigen TestNews article24 Dec 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia17 Dec 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia17 Dec 2020 -
 From: LSTMNew spray, co-developed with the British Army and tested by LSTM, kills coronavirus in under 60 secondsNews article17 Dec 2020
From: LSTMNew spray, co-developed with the British Army and tested by LSTM, kills coronavirus in under 60 secondsNews article17 Dec 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia10 Dec 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia10 Dec 2020 -
From: LSTMOxford Covid vaccine 'safe and effective', Lancet study showsNews article9 Dec 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia8 Dec 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia8 Dec 2020 -
 From: LSTMEvidence of exposure to SARS-CoV-2 in cats and dogs from households in ItalyNews article4 Dec 2020
From: LSTMEvidence of exposure to SARS-CoV-2 in cats and dogs from households in ItalyNews article4 Dec 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia3 Dec 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia3 Dec 2020 -
 From: LSTMLSTM’s Professor Miriam Taegtmeyer and Dr Tom Wingfield in The Conversation: ‘We will not forget our colleagues who have died’News article2 Dec 2020
From: LSTMLSTM’s Professor Miriam Taegtmeyer and Dr Tom Wingfield in The Conversation: ‘We will not forget our colleagues who have died’News article2 Dec 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia30 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia30 Nov 2020 -
 From: LSTMLSTM Annual Report and Financial Statements 2019-2020News article26 Nov 2020
From: LSTMLSTM Annual Report and Financial Statements 2019-2020News article26 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia26 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia26 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia20 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia20 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia19 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia19 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia12 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia12 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Sara BeggMedia11 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Sara BeggMedia11 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia10 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia10 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia5 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia5 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia4 Nov 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia4 Nov 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia29 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia29 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia28 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia28 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia26 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia26 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia22 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia22 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia19 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia19 Oct 2020 -
 From: LSTMWorld Mental Health Day 2020: one LSTM student’s research on mental health amongst health workers in Sierra LeoneNews article9 Oct 2020
From: LSTMWorld Mental Health Day 2020: one LSTM student’s research on mental health amongst health workers in Sierra LeoneNews article9 Oct 2020 -
 From: LSTMWorld Mental Health Day 2020. Protecting the mental health of the global health workforce during COVID-19: Lessons learned from the Ebola outbreakNews article9 Oct 2020
From: LSTMWorld Mental Health Day 2020. Protecting the mental health of the global health workforce during COVID-19: Lessons learned from the Ebola outbreakNews article9 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia1 Oct 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia1 Oct 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia29 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia29 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia24 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia24 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia23 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia23 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia23 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia23 Sep 2020 -
 From: LSTMAGILE trial now recruiting patients for efficacy of COVID-19 treatmentsNews article18 Sep 2020
From: LSTMAGILE trial now recruiting patients for efficacy of COVID-19 treatmentsNews article18 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia17 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia17 Sep 2020 -
 From: LSTMLSTM researchers find an easy alternative to nasal and throat swabbing for SARS-CoV-2 DiagnosisNews article14 Sep 2020
From: LSTMLSTM researchers find an easy alternative to nasal and throat swabbing for SARS-CoV-2 DiagnosisNews article14 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia14 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Nick BeechingMedia14 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia11 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia11 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia9 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia9 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia8 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia8 Sep 2020 -
 From: LSTMPaul Garner on long haul covid-19—Don’t try to dominate this virus, accommodate itBlog4 Sep 2020
From: LSTMPaul Garner on long haul covid-19—Don’t try to dominate this virus, accommodate itBlog4 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia3 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia3 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia2 Sep 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia2 Sep 2020 -
 From: LSTMBMJ Webinar: 'Long COVID: diagnosis, management, prognosis'Event3 Sep 2020
From: LSTMBMJ Webinar: 'Long COVID: diagnosis, management, prognosis'Event3 Sep 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia27 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia27 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Senior Clinical Lecturer Dr Tom WingfieldMedia24 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Senior Clinical Lecturer Dr Tom WingfieldMedia24 Aug 2020 -
From: LSTMWould you like to volunteer to take part in COVID -19 Vaccine Research?News article20 Aug 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia20 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia20 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia18 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia18 Aug 2020 -
 From: LSTMCall for volunteers: COVID-LIV Virology StudyNews article17 Aug 2020
From: LSTMCall for volunteers: COVID-LIV Virology StudyNews article17 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia17 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia17 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia13 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia13 Aug 2020 -
From: LSTMCOVID-19 campaign to reach millions in 9 African countriesNews article11 Aug 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Dr Aitor Casas-SanchezMedia11 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Aitor Casas-SanchezMedia11 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Rachel ByrneMedia4 Aug 2020
From: LSTMBBC Radio Merseyside with LSTM's Rachel ByrneMedia4 Aug 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia30 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia30 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Ian PattersonMedia28 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Ian PattersonMedia28 Jul 2020 -
 From: LSTMEvidence of exposure to SARS-CoV-2 in cats and dogs from households in ItalyNews article27 Jul 2020
From: LSTMEvidence of exposure to SARS-CoV-2 in cats and dogs from households in ItalyNews article27 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia23 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia23 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia21 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom WingfieldMedia21 Jul 2020 -
 From: LSTMSaliva offers a non-invasive alternative to upper respiratory swabs for COVID-19 diagnosisNews article17 Jul 2020
From: LSTMSaliva offers a non-invasive alternative to upper respiratory swabs for COVID-19 diagnosisNews article17 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia17 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Emily AdamsMedia17 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia16 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia16 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia14 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia14 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia13 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia13 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia9 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia9 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia8 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia8 Jul 2020 -
 From: LSTMLSTM's Prof. Paul Garner on BBC Radio 5 LiveMedia6 Jul 2020
From: LSTMLSTM's Prof. Paul Garner on BBC Radio 5 LiveMedia6 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Sarah Lewis NewtonMedia6 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Sarah Lewis NewtonMedia6 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia2 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia2 Jul 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia1 Jul 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Angela ObasiMedia1 Jul 2020 -
 From: LSTMMLW Programme opens oxygen concentration plant in Blantyre, MalawiNews article29 Jun 2020
From: LSTMMLW Programme opens oxygen concentration plant in Blantyre, MalawiNews article29 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Janet HemingwayMedia29 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Janet HemingwayMedia29 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Nurse Kate BradfieldMedia26 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Nurse Kate BradfieldMedia26 Jun 2020 -
From: LSTMLSTM-led consortium receives £18.6m from UKRINews article26 Jun 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia25 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia25 Jun 2020 -
 From: LSTMPaul Garner: Covid-19 at 14 weeks—phantom speed cameras, unknown limits, and harsh penaltiesBlog23 Jun 2020
From: LSTMPaul Garner: Covid-19 at 14 weeks—phantom speed cameras, unknown limits, and harsh penaltiesBlog23 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Ian PattersonMedia23 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Ian PattersonMedia23 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Laura JeffreysMedia19 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Laura JeffreysMedia19 Jun 2020 -
 From: LSTMLSTM's third virtual symposium on Global Health Research & Vaccination: Private sector partnership, research and accelerating the development of new toolsNews article18 Jun 2020
From: LSTMLSTM's third virtual symposium on Global Health Research & Vaccination: Private sector partnership, research and accelerating the development of new toolsNews article18 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia17 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia17 Jun 2020 -
 From: LSTMLMICs will face 'extreme strain' on health systems due to COVID-19 despite younger populationsNews article12 Jun 2020
From: LSTMLMICs will face 'extreme strain' on health systems due to COVID-19 despite younger populationsNews article12 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Carla Solórzano-GonzalezMedia12 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Carla Solórzano-GonzalezMedia12 Jun 2020 -
 From: LSTMUnique Urwerk watch raises over £33,000 for LSTM's COVID-19 researchNews article12 Jun 2020
From: LSTMUnique Urwerk watch raises over £33,000 for LSTM's COVID-19 researchNews article12 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia11 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia11 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia9 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Tom FletcherMedia9 Jun 2020 -
 From: LSTMMore than 250 Merseyside volunteers vaccinated in COVID-19 Oxford vaccine trialNews article8 Jun 2020
From: LSTMMore than 250 Merseyside volunteers vaccinated in COVID-19 Oxford vaccine trialNews article8 Jun 2020 -
 From: LSTMCOVID-19 antibody test validated by LSTM researchers ready for manufacture and distributionNews article4 Jun 2020
From: LSTMCOVID-19 antibody test validated by LSTM researchers ready for manufacture and distributionNews article4 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia4 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia4 Jun 2020 -
 From: LSTMScreening of licensed drugs to potentially treat COVID-19Blog3 Jun 2020
From: LSTMScreening of licensed drugs to potentially treat COVID-19Blog3 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Daniela FerreiraMedia3 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Daniela FerreiraMedia3 Jun 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Janet HemingwayMedia1 Jun 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Janet HemingwayMedia1 Jun 2020 -
 From: LSTMCOVID-19 Oxford Vaccine Trial: LSTM is currently recruiting volunteersNews article28 May 2020
From: LSTMCOVID-19 Oxford Vaccine Trial: LSTM is currently recruiting volunteersNews article28 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia28 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia28 May 2020 -
From: LSTM'Access, equity and delivery': LSTM’s second virtual symposium on Global Health Research and VaccinationNews article28 May 2020
-
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia27 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia27 May 2020 -
 From: LSTMLSTM named as a site for Phase III of the Oxford COVID-19 vaccine human trialsNews article22 May 2020
From: LSTMLSTM named as a site for Phase III of the Oxford COVID-19 vaccine human trialsNews article22 May 2020 -
 From: LSTMLSTM's Dr Andrea Collins on BBC Radio MerseysideMedia22 May 2020
From: LSTMLSTM's Dr Andrea Collins on BBC Radio MerseysideMedia22 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia21 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia21 May 2020 -
 From: LSTMLSTM's Dr Lee Haines on BBC Radio MerseysideMedia20 May 2020
From: LSTMLSTM's Dr Lee Haines on BBC Radio MerseysideMedia20 May 2020 -
 From: LSTMCovid19 and fatigue - a game of snakes and laddersBlog20 May 2020
From: LSTMCovid19 and fatigue - a game of snakes and laddersBlog20 May 2020 -
 From: LSTMLSTM's Dr Shaun Pennington on BBC Radio MerseysideMedia19 May 2020
From: LSTMLSTM's Dr Shaun Pennington on BBC Radio MerseysideMedia19 May 2020 -
 From: LSTMTowards ending preventable deaths: Global health research and vaccinations in the context of emerging pandemics – answering your questions from our first global symposiumBlog18 May 2020
From: LSTMTowards ending preventable deaths: Global health research and vaccinations in the context of emerging pandemics – answering your questions from our first global symposiumBlog18 May 2020 -
From: LSTMPaul Garner: ‘The virus is causing lots of immunological changes in the body, lots of pathology that we don’t yet understand.’Blog15 May 2020
-
 From: LSTMLSTM led study: While adapting to COVID-19 can Liverpool’s health system learn from the West African Ebola outbreak?News article15 May 2020
From: LSTMLSTM led study: While adapting to COVID-19 can Liverpool’s health system learn from the West African Ebola outbreak?News article15 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia15 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Kevin MortimerMedia15 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia14 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia14 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Adam RobertsMedia13 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Adam RobertsMedia13 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia7 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia7 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Samantha DonnellanMedia6 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Samantha DonnellanMedia6 May 2020 -
 From: LSTMPaul Garner: For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustionBlog5 May 2020
From: LSTMPaul Garner: For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustionBlog5 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Imelda BatesMedia4 May 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Imelda BatesMedia4 May 2020 -
 From: LSTMVirtual Race to Malawi ChallengeNews article1 May 2020
From: LSTMVirtual Race to Malawi ChallengeNews article1 May 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia30 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia30 Apr 2020 -
From: LSTMLSTM begins series of virtual vaccine research symposia with global guests and new funding pledgeNews article30 Apr 2020
-
 From: LSTMMy letter to LiverpoolBlog29 Apr 2020
From: LSTMMy letter to LiverpoolBlog29 Apr 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Sean TomlinsonMedia28 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Sean TomlinsonMedia28 Apr 2020 -
 From: LSTMLSTM & World Immunization Week 2020News article27 Apr 2020
From: LSTMLSTM & World Immunization Week 2020News article27 Apr 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Dr Andrea CollinsMedia24 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Dr Andrea CollinsMedia24 Apr 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia23 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Professor Bertie SquireMedia23 Apr 2020 -
 From: LSTMGrowing support for LSTM’s COVID-19 Response and Resilience Fund in MalawiNews article22 Apr 2020
From: LSTMGrowing support for LSTM’s COVID-19 Response and Resilience Fund in MalawiNews article22 Apr 2020 -
 From: LSTMAn open letter from colleagues at the Malawi-Liverpool-Wellcome Trust Clinical Research ProgrammeNews article21 Apr 2020
From: LSTMAn open letter from colleagues at the Malawi-Liverpool-Wellcome Trust Clinical Research ProgrammeNews article21 Apr 2020 -
 From: LSTMLSTM’s 3D printers reallocated to print face shields for NHS frontline staffNews article21 Apr 2020
From: LSTMLSTM’s 3D printers reallocated to print face shields for NHS frontline staffNews article21 Apr 2020 -
 From: LSTMMeet the team of our CL3 containment laboratories conducting some of LSTM's Covid-19 researchBlog20 Apr 2020
From: LSTMMeet the team of our CL3 containment laboratories conducting some of LSTM's Covid-19 researchBlog20 Apr 2020 -
 From: LSTMCovid-19 & Maternal Health: Are we facing a new normal?Event16 Apr 2020
From: LSTMCovid-19 & Maternal Health: Are we facing a new normal?Event16 Apr 2020 -
 From: LSTMBBC Radio Merseyside with LSTM's Senior Clinical Lecturer Dr Tom WingfieldMedia14 Apr 2020
From: LSTMBBC Radio Merseyside with LSTM's Senior Clinical Lecturer Dr Tom WingfieldMedia14 Apr 2020 -
From: LSTMLSTM and Pfizer Inc. announce two new studies on the interaction between S. pneumoniae and SARS-CoV-2News article9 Apr 2020
-
 From: LSTM“To an unpredictable future!” - in times of DTM&H - Corona editionBlog8 Apr 2020
From: LSTM“To an unpredictable future!” - in times of DTM&H - Corona editionBlog8 Apr 2020 -
 From: LSTMBMJ editorial: LSTM clinicians call for rapid near-patient testing for COVID-19News article8 Apr 2020
From: LSTMBMJ editorial: LSTM clinicians call for rapid near-patient testing for COVID-19News article8 Apr 2020 -
 From: LSTMLSTM researchers look at the impact of COVID-19 on healthcare workersNews article7 Apr 2020
From: LSTMLSTM researchers look at the impact of COVID-19 on healthcare workersNews article7 Apr 2020 -
 From: LSTMBBC Radio Merseyside's SciFri with Rachel Byrne MRC/DTP PhD candidateMedia3 Apr 2020
From: LSTMBBC Radio Merseyside's SciFri with Rachel Byrne MRC/DTP PhD candidateMedia3 Apr 2020 -
 From: LSTMLSTM key partner in publishing K4D COVID-19 Health Evidence SummariesNews article3 Apr 2020
From: LSTMLSTM key partner in publishing K4D COVID-19 Health Evidence SummariesNews article3 Apr 2020 -
 From: LSTMLSTM joins global coalition to accelerate research on the prevention and treatment of COVID-19 in low- and middle-income countriesNews article2 Apr 2020
From: LSTMLSTM joins global coalition to accelerate research on the prevention and treatment of COVID-19 in low- and middle-income countriesNews article2 Apr 2020 -
 From: LSTMCOVID-19 and disability: virtual Q&AEvent6 Apr 2020
From: LSTMCOVID-19 and disability: virtual Q&AEvent6 Apr 2020 -
 From: LSTMAntibiotic resistance could lead to more COVID-19 deathsBlog2 Apr 2020
From: LSTMAntibiotic resistance could lead to more COVID-19 deathsBlog2 Apr 2020 -
 From: LSTMLSTM launches fundraising appeal to support COVID-19 research and response in MalawiNews article1 Apr 2020
From: LSTMLSTM launches fundraising appeal to support COVID-19 research and response in MalawiNews article1 Apr 2020 -
 From: LSTMPandemic Preparedness PurseBlog1 Apr 2020
From: LSTMPandemic Preparedness PurseBlog1 Apr 2020 -
 From: LSTMLSTM's Respiratory Clinical Research Group conducts key COVID-19 research in patients & healthcare workersNews article31 Mar 2020
From: LSTMLSTM's Respiratory Clinical Research Group conducts key COVID-19 research in patients & healthcare workersNews article31 Mar 2020 -
 From: LSTMOpen data and software to support the COVID-19 response in AfricaNews article30 Mar 2020
From: LSTMOpen data and software to support the COVID-19 response in AfricaNews article30 Mar 2020 -
 From: LSTMLSTM begins the validation process for the Mologic COVID-19 point-of-need diagnostic testNews article27 Mar 2020
From: LSTMLSTM begins the validation process for the Mologic COVID-19 point-of-need diagnostic testNews article27 Mar 2020 -
 From: LSTMBlog: Don’t get complacent AMR; we’ll be backNews article25 Mar 2020
From: LSTMBlog: Don’t get complacent AMR; we’ll be backNews article25 Mar 2020 -
From: LSTMDon’t get complacent AMR; we’ll be backBlog25 Mar 2020
-
 From: LSTM“Antibodies will make a significant contribution to design effective control strategies for COVID-19”News article24 Mar 2020
From: LSTM“Antibodies will make a significant contribution to design effective control strategies for COVID-19”News article24 Mar 2020 -
 From: LSTMA Tale of Two Pandemics: a plea on World TB DayNews article24 Mar 2020
From: LSTMA Tale of Two Pandemics: a plea on World TB DayNews article24 Mar 2020 -
 From: LSTMCOVID-19: LSTM's Dr Tom Wingfield answers questions from LSTM's overseas partnersNews article20 Mar 2020
From: LSTMCOVID-19: LSTM's Dr Tom Wingfield answers questions from LSTM's overseas partnersNews article20 Mar 2020 -
 From: LSTMLSTM researchers work on new diagnostics for COVID-19News article12 Mar 2020
From: LSTMLSTM researchers work on new diagnostics for COVID-19News article12 Mar 2020 -
 From: LSTMBMJ Best Practice monograph: COVID-19News article20 Feb 2020
From: LSTMBMJ Best Practice monograph: COVID-19News article20 Feb 2020
COVID-19 Advocacy
Building Back Better for All
The Briefing tackles the urgent and topical issue of the disproportionate impact of COVID-19 on racially minoritised groups. It highlights that this impact is “not only directly felt in terms of health outcomes but compounds and is compounded by unequal social impacts in housing, education, employment social care/welfare and criminal justice”.As part of a series of COVID-10 Policy Briefings, commissioned by University of Liverpool’s Heseltine Institute to support civic leaders and policy makers in their response to the challenges of the COVID-19 pandemic, LSTM’s Dr Angela Obasi was co-author on the Policy Briefing ‘Racial inequalities and COVID-19: Building Back Better for All.
The Briefing summarises the adverse impacts on Black, Asian and other racially minoritised people and outlines the need for more rigorous exploration and analysis of policy making and policy impacts with regard to racial equity.
The authors highlight the importance of meaningful and well-resourced multi-sector partnership in the design and implementation of strategies for recovery and the need for such strategies to be co-produced with communities to help counteract the impact of racial inequalities.
The recommendations are designed to support Liverpool civic leaders and policy makers to take the opportunity that recovery programming provides to not merely to return to “normal” but to actively remedy inequalities and truly build back better for all.

COVID-19 Clinical Research Coalition

LSTM Director, Professor David Lalloo, joined a group of scientists, physicians, funders and policy makers from over 70 institutions from over 30 countries who launched an international coalition to respond to COVID-19 in resource-poor settings.
The COVID-19 Clinical Research Coalition aims to accelerate desperately needed COVID-19 research in those areas where the virus could wreak havoc on already-fragile health systems and cause the greatest health impact on vulnerable populations.
In a Comment published in The Lancet, the members of the coalition argue that international research collaboration and coordination is needed urgently to support African, Latin American, Eastern European, and certain Asian countries to respond effectively to the worsening pandemic and speed up research adapted to resource-limited settings.