The Malaria Epidemiology Unit of the Clincial Sciences Department was created in 2003 with the arrival of Prof Feiko ter Kuile, a senior clinical epidemiologist who previously worked with the Malaria Branch of the US-based Centers for Disease Control and Prevention (CDC). This unit has seen a steady growth and currently consists of 3 clinical epidemiologists (Prof Feiko ter Kuile, Dr Anja Terlouw and Dr Annemieke van Eijk), a public health scientist (Dr Jenny Hill), two research assistants, one in pharmaco-epidemiology (Dr Stephanie Dellicour) and one in epidemiology (Carole Khairallah), 6 PhD students and administrative staff Helen Wong.
The unit is involved in teaching, provides technical assistance, and has a strong emphasis on research into the treatment and prevention of malaria in endemic countries. In order to ensure that new ways of preventing and treating malaria are discovered and implemented as speedily and effectively as possible, international research efforts are increasingly focusing on larger multidisciplinary and multi-centre research and evaluation. This is reflected in the unit's research agenda which is part of several productive international alliances that aim to improve the control of malaria in young children and pregnant women. The majority of our research collaborations are based in western Kenya and Malawi.
Research activities
The Malaria Epidemiology Unit hosts the international co-ordinating centre for the Malaria in Pregnancy (MiP) Consortium. LSTM plays a key role in the research portfolio of the MiP Consortium.
The Unit also has a long-term research collaboration with the US-based Centers for Disease Control and Prevention (CDC) to jointly conduct applied research to identify, evaluate, and implement interventions for the control of malaria in high risk groups in Africa and Asia and work in a complimentary way towards the targets set by the Roll Back Malaria (RBM) Initiative.
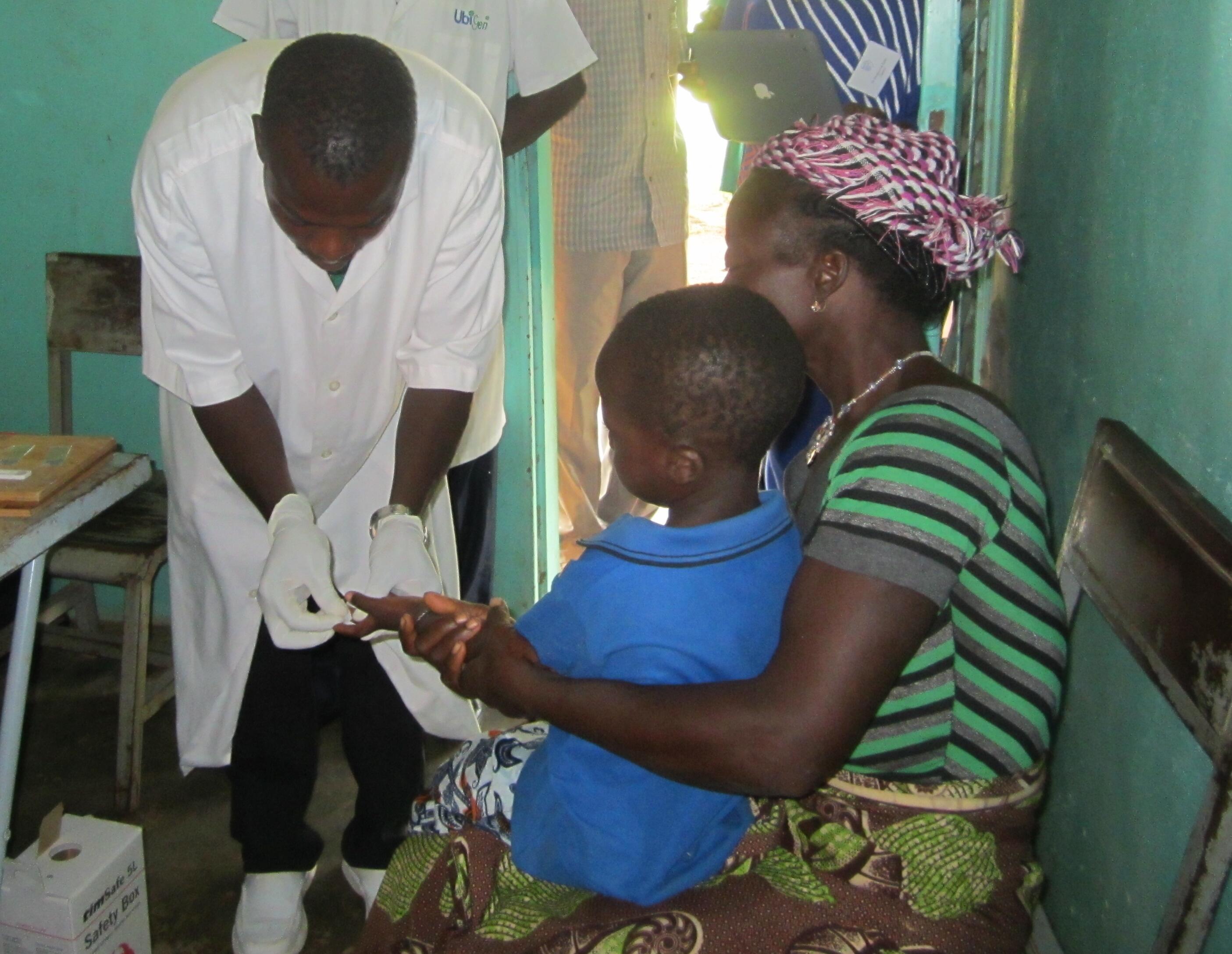
Dr. Dianne (Anja) Terlouw is involved in two large community-based trials on the safety and effectiveness of malaria treatment funded by the ACT Consortium and EDCTP. Specifically the trials aim to evaluate the safety and effectiveness of ACTs when used repeatedly in young children over a three year follow-up period, when dosed by age instead of weight, and when used in HIV+ adults on anti-retroviral treatment.
Dr. Terlouw and Prof. ter Kuile are also working on a methodology to define age-based dosing regimens for the treatment of malaria that aim to maximize efficacy and minimize toxicity, while taking ease-of-use into account using weight-for-age reference data from target populations in malaria endemic countries. The method has been successfully applied to determine the optimal tablet strength and/or corresponding age-based dose regimen of a new, user-friendly, blister-packaged fixed dose combination of artesunate and amodiaquine and mefloquine-artesunate developed by Drugs for Neglected Diseases Initiative (DNDi, Geneva). This pharmaco-epidemiological tool could extend to a wide range of drugs used for neglected diseases in the tropics.
Prof. ter Kuile is working closely with Dr. Michael Boele van Hensbroek from the University of Amsterdam, and Dr. Kamija Phiri from the Malawi-Liverpool-Welcome Trust Research Laboratories at the College of Medicine in Blantyre, Malawi, to evaluate the efficacy of Intermittent Preventive Therapy in the post-discharge (IPTpd) management of young Malawian children with severe anaemia.
