The Effect of Live Attenuated Inactivated Influenza Vaccine on Experimental Human Pneumococcal Colonisation (LAIV in EHPC)
Vaccination to protect against Flu virus is very important. We want to test the effect of a new licenced nasal flu vaccine upon the carriage of pneumococcal bacteria in the nose.
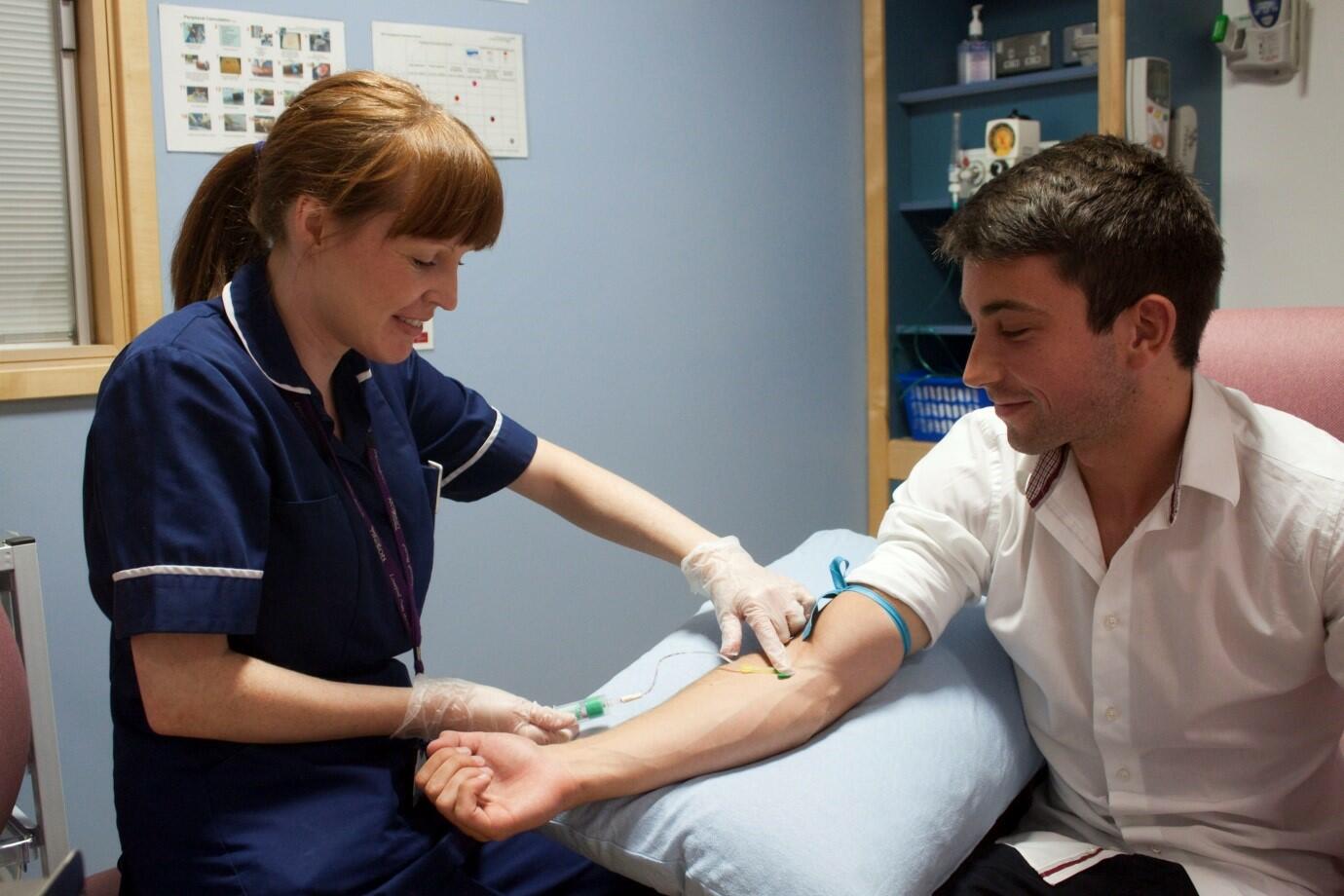
Healthy, non-smoking volunteers are invited to participate in research related to flu vaccinations and Streptococcus pneumoniae (a cause of pneumonia, blood infection, and meningitis). Volunteers will be seen as an outpatient and they will be invited to give blood, throat swabs and nasal samples. As an optional part of the study, volunteers could also undergo a bronchoscopy (camera test into the lungs).
Who can take part?
You will be eligible to participate in this study provided that you have/are:
- capacity to give informed consent
- aged 18-50 yrs - ages chosen to minimise the risk of pneumococcal infection
- fluent in English - to ensure a comprehensive understanding of the research project and their proposed involvement, in order to minimise any communication issues to maximise participant safety.
Who cannot take part?
People who have/are:
- currently involved in another study unless observational or in follow-up phase (non-interventional)
- received any influenza vaccine over the last 2 years
- egg allergy (as per influenza vaccines patient leaflet)
- previous significant adverse reaction to any vaccination/immunisation
- close contact with at risk individuals (children under 5years, immunosuppressed adults, elderly, chronic ill health) – to minimise risk of pneumococcal transmission and transmission of virus for those receiving the LAIV
- current regular smoker (smokes daily)
- significant smoking history [defined as someone who has previously smoked more than 20 cigarettes per day for 10 years or the equivalent (>10 pack yrs)] – to minimise risk of bronchoscopy or pneumococcal disease
- asthma (on regular medication) or respiratory disease – to minimise risk of bronchoscopy or pneumococcal disease
- pregnant - to minimise the risk of pneumococcal disease
- women of child-bearing potential (WOCBP) who are not deemed to have sufficient, effective birth control in place for 1 month prior to vaccination and 1 month after the final vaccination
- allergic to penicillin/amoxicillin/gentamicin
- taking medication that may affect the immune system in any way e.g. steroids, steroid nasal spray
- regularly taking acetylsalicylic acid (aspirin) - as per LAIV guidance to reduce the risk of Reye’s syndrome
- been involved in a clinical trial involving experimental human pneumococcal carriage in the last 3 years
- unable to give fully informed consent
- current acute severe febrile illness - to avoid vaccination and inoculation in participants that may have current infection.
This study is now closed. View the results