The need for new tools to prevent vector borne diseases by targeting the insects that transmit the pathogens is widely recognised but there is less consensus on how these new tools should be evaluated to determine their public health value.
One of the largest ever trials of vector control tools is ongoing in Uganda; the LLIN Evaluation in Uganda Project (LLINEUP) is employing an innovative study design, embedding a randomised controlled trial within a routine bednet distribution campaign to evaluate the public health value of nets containing the traditional pyrethroid insecticides plus the synergist piperonyl butoxide (PBO). The scale of the trial affords a unique opportunity to evaluate PBO nets across different epidemiological settings. The large-scale trial embedded within could be a paradigm for future assessment of malaria control interventions. It may also help inform national policy on bednet use.
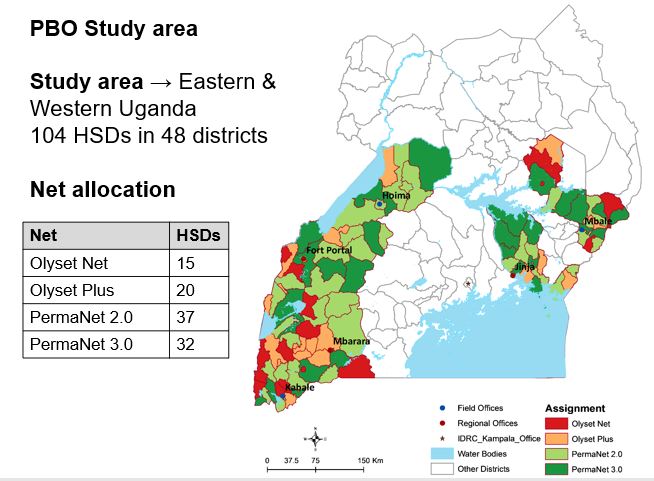
In total 104 health sub-districts in 48 districts of Eastern and Western Uganda were selected for the trial. Of these 104 health sub-districts (HSDs), 38 are located in the Eastern region and the remaining 66 in the Western region. The study region encompasses approximately half the total area of Uganda and includes a wide range of epidemiological settings with varying levels of malaria transmission and insecticide resistance. The study clusters have been assigned to one of four groups. In each area households receive one of four LLINs that have been approved by the World Health Organization.
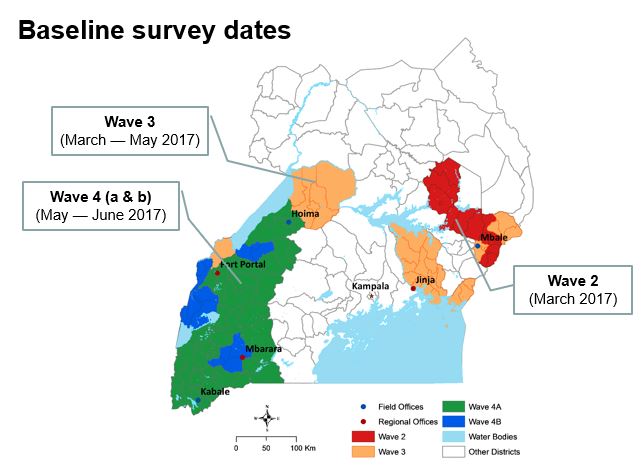
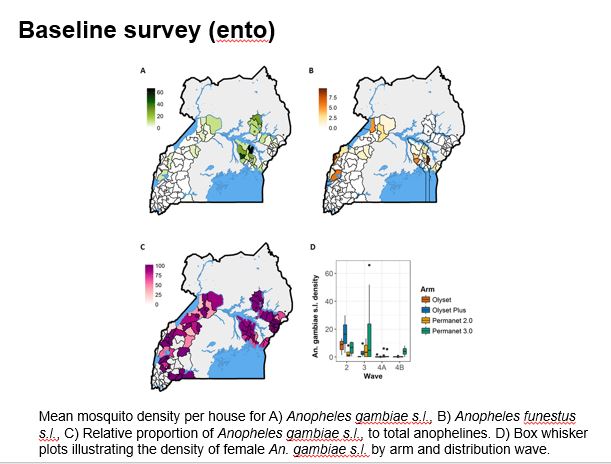
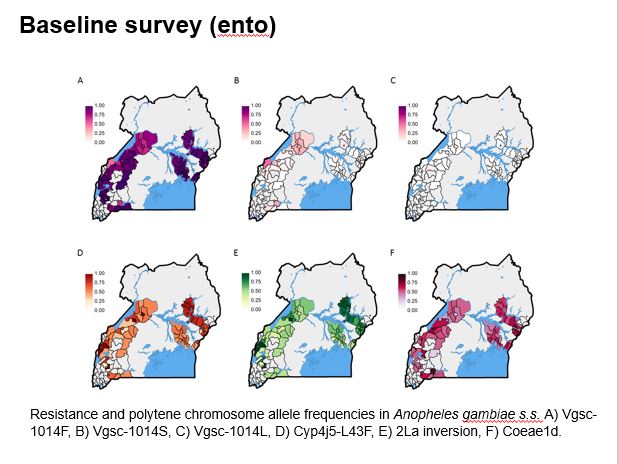
The aim of this study is to determine whether parasite prevalence will be lower in intervention clusters (HSDs) randomised to receive the next generation PBO-treated LLINs, than in control clusters (HSDs randomised to conventional nets). The evaluation is by repeated cross-sectional community surveys to gather information on net survivorship and use, and parasite prevalence in children over 5 years of age, entomological surveillance for insecticide resistance monitoring, and assessment of net durability and bio-efficacy at 12 months. The primary outcome of the trial is parasite prevalence as measured by microscopy in the cross-sectional surveys at baseline, 6, 12 and 18 months.
Project partners are The Republic of Uganda Ministry of Health; the Infectious Diseases Research Collaboration, Uganda; University of California San Francisco and the London School of Hygiene and Tropical Medicine.
Funding for the trial comes from the Against Malaria Foundation; the Department for International Development and the Innovative Vector Control Consortium.
 |
 |