
From time-to-time we all take medication, be it for a headache, stomach-ache, or to help keep our cholesterol under control. These medications are typically easy to obtain, inexpensive, and taken orally as a tablet/capsule. But imagine if your headache medication had to be given at a hospital via an intravenous injection, or if your potentially lifesaving blood pressure medication cost you factors of magnitude more than your annual salary. These issues are the current reality for victims of snakebite – a neglected tropical disease that kills approximately 138,000 people every year – in many tropical and developing nations, such as those in sub-Saharan Africa 1.
Here at the Centre for Snakebite Research & Interventions (CSRI) in Liverpool, UK, one of our research areas of interest is in the development of new small molecule-based snakebite therapies that could be taken orally and that are significantly cheaper to produce than currently used antivenoms. Antivenoms are expensive biologic therapies to produce, can induce severe adverse events, and must be injected by a clinical healthcare professional. This means that a snakebite victim must reach their nearest healthcare centre to receive lifesaving antivenom; a journey that can take hours or even days in certain parts of rural Africa in a situation where minutes matter. In addition, small clinics in particularly remote communities often do not even have the proper antivenom available, meaning there is little the healthcare workers can do to help the victim 1. If an orally active small molecule-based snakebite treatment existed as a tablet, it could be kept in any rural village or home in case of an emergency. This tablet could be taken within minutes of a snakebite occurrence, buying the victim precious time to get to hospital for antivenom and potentially saving their life. In a recent paper published in Science Translational Medicine led by Dr Laura-Oana Albulescu and Prof Nick Casewell from the CSRI, they explored the possibility that an oral medication known as DMPS (full chemical name: 2,3-dimercapto-1-proanesulfonic acid; trade name: Dimaval) could be repurposed as a treatment for certain types of snakebite 2.

DMPS is already approved as a treatment for heavy metal poisoning. DMPS contains two strongly nucleophilic sulphur atoms, which allows it to tightly ‘grab’ positively charged metal ions and remove them from a person’s circulation. This is called chelation therapy, a wordage that derives from the Greek word for claw, ‘chele’. You can think of chelation exactly like a lobster claw, grabbing onto something and holding on tightly from at least two points.
A powerful family of proteins found in many snake venoms, called snake venom metalloproteinases (SVMPs), depend on the metal ion zinc to induce their toxic effects in animals or people. SVMPs make up a large portion of the venom of a particularly dangerous genus of snakes called Echis, otherwise known as saw-scaled or carpet vipers. These snakes are very common in sub-Saharan Africa, the Middle East and India, and despite their small size are among the world’s deadliest snakes. This is not because their venom is the most potent or rapid, but because of how many bites they inflict. For example, E. ocellatus, a specific species of Echis, are found in high numbers in western sub-Saharan Africa and often in farmers’ fields. This unfortunately results in many unwitting farmers being bitten after accidentally stepping on or near these snakes. The quicker the snakebite victim can receive antivenom the higher their chances of survival, meaning that time is of the essence for them to get to hospital to receive treatment. Therefore, an oral therapeutic that could be taken shortly after a snakebite is received and that reduces the venom’s toxicity, thus buying them time to reach hospital, could save many lives.

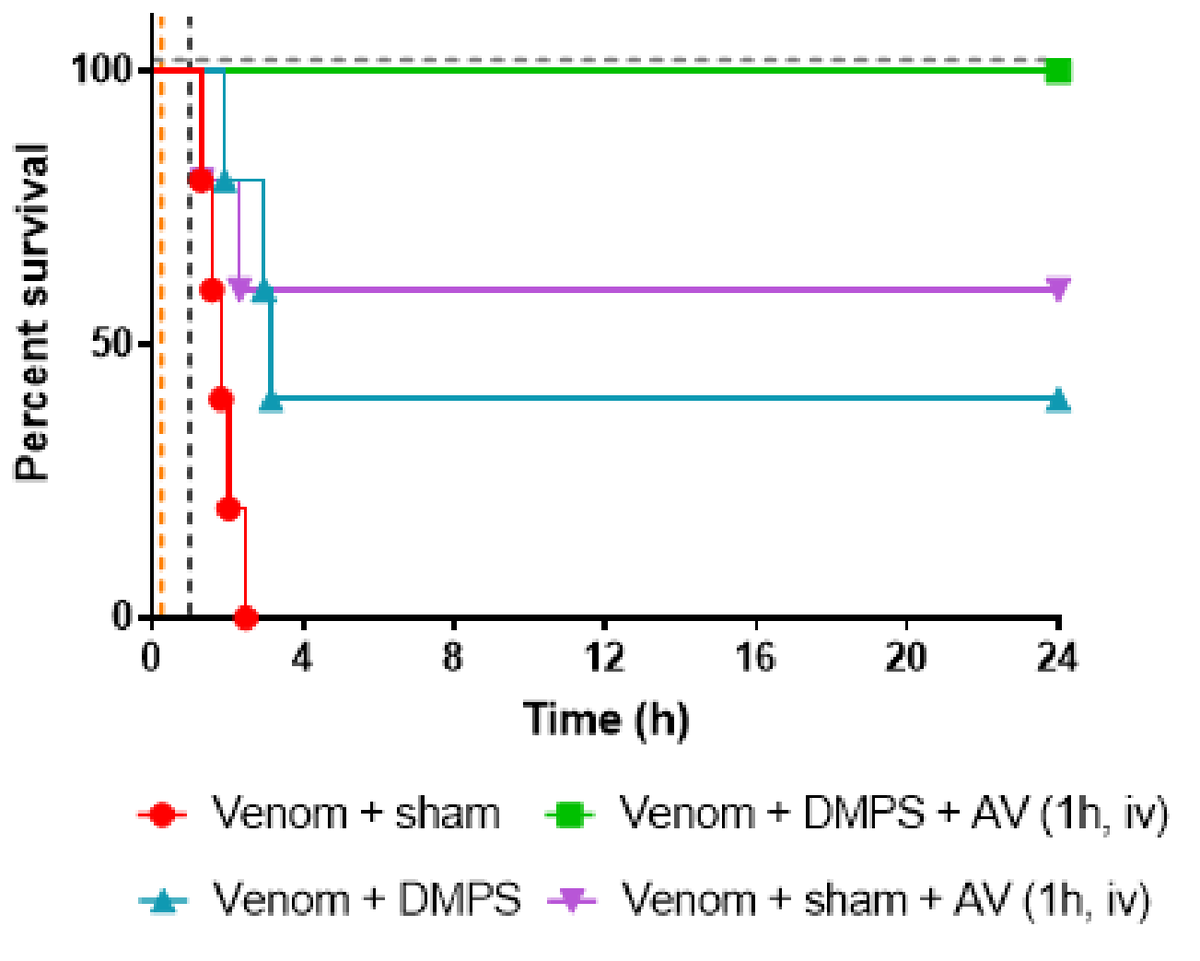
It has previously been suggested in the literature that if zinc were removed from the equation using chelation therapy, then perhaps venoms containing high levels of SVMPs would become less toxic. In preclinical studies, Dr Albulescu tested a number of potential chelation therapies for activity against Echis venoms, with the most promising one being DMPS. When given orally 15 minutes after envenomation (to mimic a person being bitten by a snake and then taking a tablet shortly afterwards), she found that DMPS saved 40% of E. ocellatus envenomed mice versus 0% survival in envenomed mice not given any treatment. In contrast, the antivenom treatment given 1 hour after envenomation (to mimic a snakebite victim receiving antivenom at a hospital after they are bitten) saved 60% of mice. Excitingly, when the mice were given DMPS orally 15 minutes after envenomation AND antivenom 1 hour after envenomation, survival was 100%! This is an incredible discovery that provides important proof-of-concept data that small molecule therapeutics given orally can reduce the lethality of snake venoms. In addition, because DMPS is currently used in humans with heavy metal poisoning, this means it has already passed all necessary clinical safety trials thereby making it much cheaper and quicker to study in human snakebite victims than if it was a ‘new chemical entity’ (the name used to describe a compound being tested as a drug but not yet approved as a drug).
One of our ultimate goals at the CSRI is to make the treatment of snakebite as simple, quick, and affordable as possible, such that a victim could obtain a tablet from a local dispensary within minutes of being bitten. The discovery that orally administered DMPS can reduce Echis venom toxicity in vivo is the first exciting step in reaching this ambitious target. Next, we will have to demonstrate whether oral treatment with DMPS can help snakebite victims clinically. If it is found to be successful in people, oral treatment with DMPS has the potential to truly revolutionize how we treat snakebite.
References:
1. Harrison, R. A., Hargreaves, A., Wagstaff, S. C., Faragher, B. & Lalloo, D. G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 3, e569 (2009).
2. Albulescu, L.-O. et al. Preclinical validation of a repurposed metal chelator as a community-based therapeutic for hemotoxic snakebite. Sci. Transl. Med. 12, eaay8314 (2020).
3. Albulescu, L.-O. et al. Preclinical validation of a repurposed metal chelator as a community-based therapeutic for hemotoxic snakebite. bioRxiv (2019) doi:10.1101/717280.