LSTM’s Professor Richard Pleass has been awarded a Wellcome Innovator Award to improve the treatment for people suffering from a range of immune-mediated neuropathies. These include chronic inflammatory polyneuropathy (CIDP), by developing and testing new synthetic versions of the treatment currently seen as the last resort option by doctors; intravenous immunoglobulin (IVIg) therapy.
The award of just under £500,000 from the Wellcome Trust will be used by Professor Pleass’ group to test the recombinant hypersialylated molecules developed by him at LSTM. Testing them in pre-clinical models of CIDP, which is a necessity for on-going commercialisation and for translation into human studies.
Professor Pleass said: “I am delighted to be able to confirm this award from Wellcome, which will help us further develop work which has the potential to bring relief to millions of people throughout the world.”
The project is scheduled to begin next week and Professor Pleass has been invited to deliver a plenary session at the 11th International Congress on Autoimmunity in Lisbon this week in relation to some of the preliminary work.
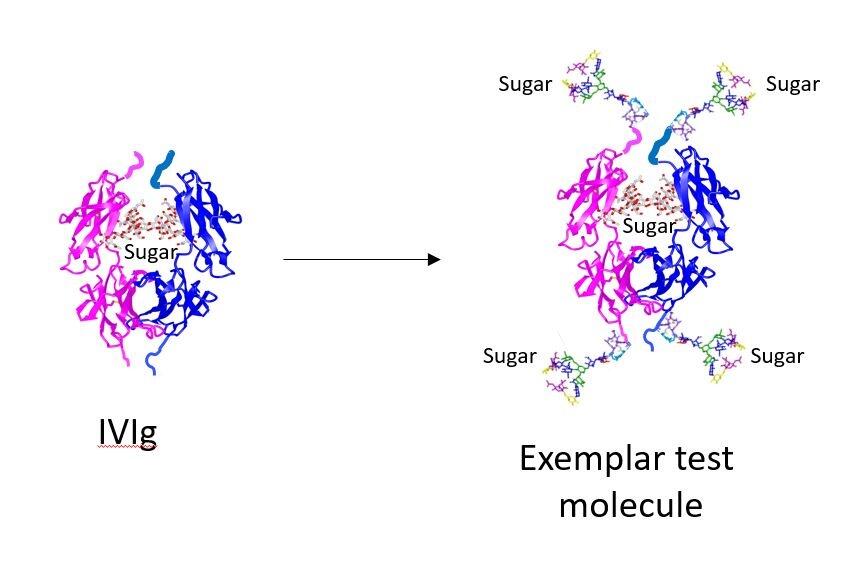
The worldwide consumption of IVIG has increased threefold since 1980, and this has impacted on supplies globally which are now critically limited meaning that patients that need the treatment are routinely deprived of it. There are significant clinical limitations in the production of IVIg which result from its dependence on human donors for manufacture, and from the fact that less than 5% of injected IVIG is therapeutically active which means that huge dosages are required to treat conditions such as CDIP. Consequently, IVIg is very expensive to produce and this, combined with adverse events was the incentive for LSTM to develop molecules with improved efficacy.
These synthetic molecules have been developed in order to overcome the challenges posed by the production of IVIg, and the team found that by adding sugars to the synthetic compound and moving their position within it, they can make up to 80% of the product active, reducing significantly the amount of product needed and therefore the cost. “We will now work with Professor Norbert Goebels at the Heinrich Heine University in Dusseldorf to test these molecules.” Continued Professor Pleass: “There is massive potential for this work, particularly as we have found a way to manufacture recombinant hypersialylated Fcs for human use means that these compounds could be produced at a fraction of the cost of IVIg and to be a great deal more effective.”
The work may also be useful for the treatment of Zika mediated neuropathies, including Guillain-Barré Syndrome, and the team at LSTM are carrying out preliminary work with Professor Alain Kohl at the University of Glasgow to determine if they are efficacious.