The editorial comment published in the American Journal of Respiratory and Critical Care Medicine hails the use of Experimental Human Pneumococcal Carriage (EHPC) model to test vaccine efficacy as ‘a milestone’ in the Investigation of Population-Wide Effects of Vaccines.
The positive comments were published in the same edition in which LSTM reported for the first time that the pneumococcal vaccine given to children in the UK reduces not only colonisation acquisition but also colonisation density. The unique point of that study was the use of the LSTM human challenge model (EHPC) to test this vaccine. The study was done in collaboration with the Clinical Research Unit of the Royal Liverpool University Hospital.
‘This is important as it opens new avenues in pneumococcal vaccine development as the EHPC model provides a way in which we can study these vaccines in the adult human population’, says one of the lead investigators Dr Daniela Ferreira of LSTM.
Current pneumococcal vaccines protect well against invasive disease in the blood but not so well against pneumonia (lung disease). Several new vaccines are under development and the impact on carriage density (quantity of bacteria in the nose) will need to be demonstrated in clinical trials in order to select the best vaccines going forward.
The editorial comment highlighted how challenge studies to test vaccines can be done in humans safely and ethically, yielding important results on nasal carriage density by using only a fraction of the time and money required for natural history based trials.
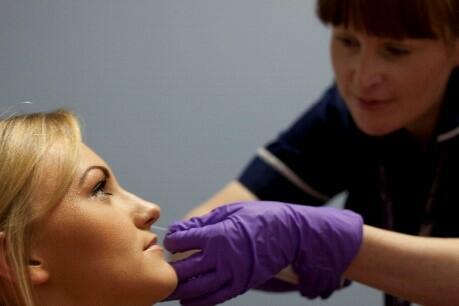
This model is the only one of its kind in the world and it can be used for vaccine development, examining pneumococcal biology, exploring mucosal immunity and host susceptibility. It involves volunteers having the pneumococcal bacteria placed directly in their nasal passage and examining their immune responses and pneumococcal biology in a controlled manner.
The research is being funded by grants from the Bill and Melinda Gates Foundation (BMGF) and the Medical Research Council (MRC).