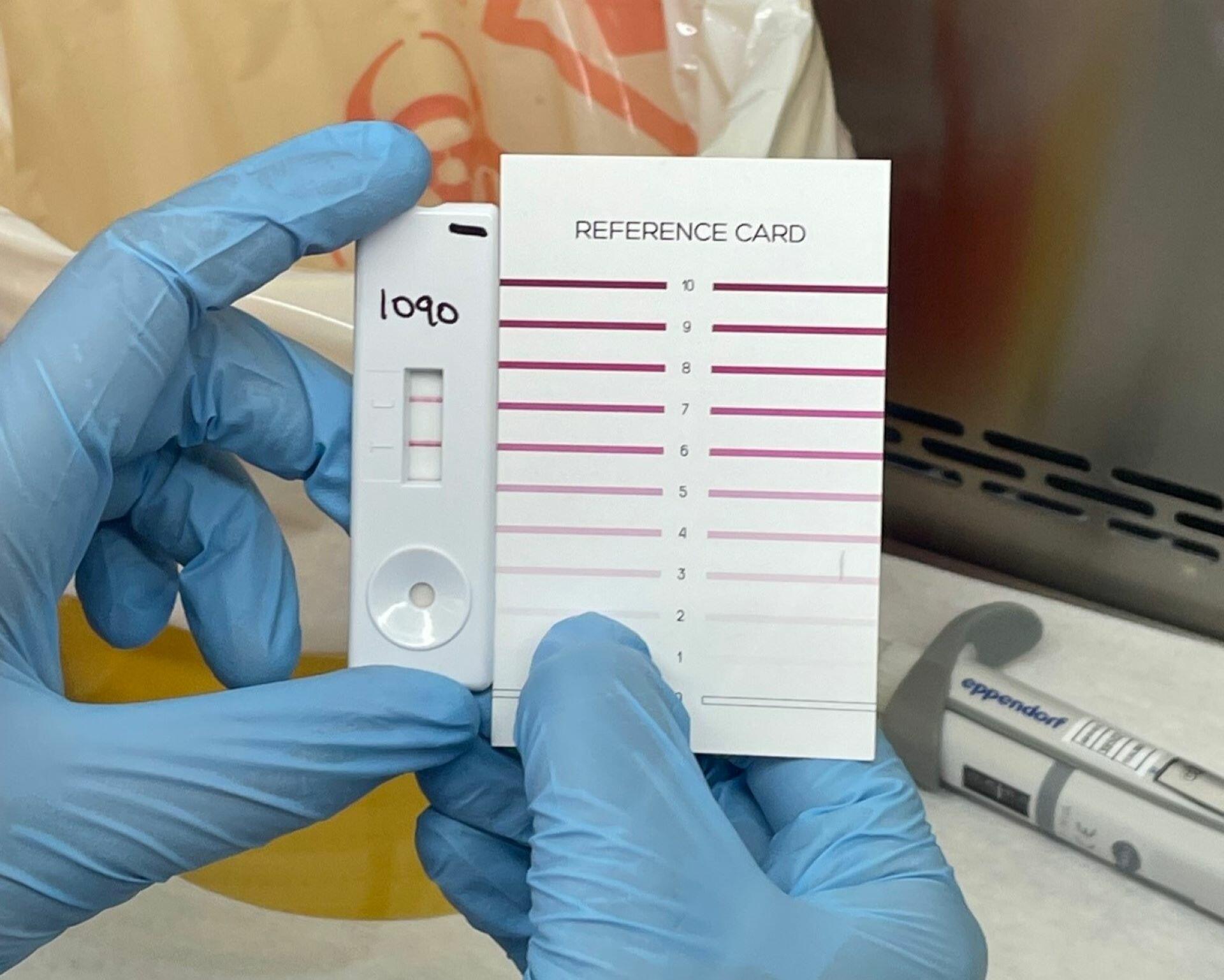
Researchers from Liverpool School of Tropical Medicine (LSTM) have developed the world's first rapid, point of care Crimean-Congo haemorrhagic fever (CCHF) test, offering faster detection of this rare but deadly disease.
The new lateral flow test enables healthcare workers to identify CCHF cases quickly, potentially saving lives by speeding up treatment and reducing the spread of outbreaks, which can have a mortality rate as high as 40%.
The development of the test was funded by NIHR and The Pandemic Institute with research carried out by LSTM, Global Access Diagnostics (GADx) led by Dr Emily Adams and clinicians and academics in Turkiye and Iraq, the countries most affected by CCHF.

Current diagnostic methods rely on PCR tests, which require laboratory analysis taking days to produce results delaying outbreak response and containment.
Transmitted primarily by tick bites, CCHF causes flu-like symptoms within a matter of days. In fatal cases, death occurs from haemorrhage, multi-organ failure and shock, usually between days five and 14 of illness.
Dr Caitlin Thompson, a Postdoctoral Research Associate at LSTM and lead researcher on the paper, said: “I’ve seen sites that have had to wait up to 10 days for PCR test results. Given the short incubation period and rapid progression of CCHF, it became clear to me that a rapid test could dramatically improve patient outcomes and allow authorities to respond swiftly to outbreaks.
“Removing the need for lab analysis also makes testing more affordable and accessible, ensuring more people can be diagnosed and treated effectively.”
Once thought to be confined to parts of Africa, CCHF has now been identified in the Middle East and Balkans, with Turkey and Iraq experiencing annual outbreaks. Recent cases in Spain and the detection of infected ticks in France suggest the disease is spreading, underscoring the urgent need for effective diagnostic tools.
The World Health Organization (WHO) has classified CCHF as a priority disease for research and development due to its epidemic potential and limited treatment options.
Dr Thompson added: “We worked closely with clinicians and academics in Turkiye and Iraq – the countries that see the most cases each year. Thanks to their support we were able to rigorously evaluate our lateral flow test over several years, using samples from the two countries.
“The results demonstrate the test’s reliability and potential to transform how we manage this deadly disease.”
LSTM is also advancing therapeutic options for CCHF through the UMIT trial. Senior Researcher Dr Tom Fletcher recently completed stage one of a pioneering clinical trial of two new treatments and has secured funding from the UK Medical Research Council (MRC) to launch a second trial called UMIT-2. If successful, this combination therapy could dramatically improve the way CCHF is treated.
Dr Cubas Atienzar, Lecturer at LSTM and lead on the CCHF diagnostic programme said: “The combination of new therapeutics and diagnostics being researched at LSTM could be a game changer in how doctors respond to CCHF and ensures they have the tools to effectively manage a large-scale outbreak, should one occur.'"
This work was funded through HPRU and TPI. Looking to the future, LSTM researchers have secured £1.3 million from the Medical Research Council to progress the prototype rapid test onto a final device with the aim of commercialisation at the end of the project.