A new study looking at deadly animal African trypanosomiasis has revealed how the parasite Trypanosoma congolense evades the immune system and drives severe disease in livestock.
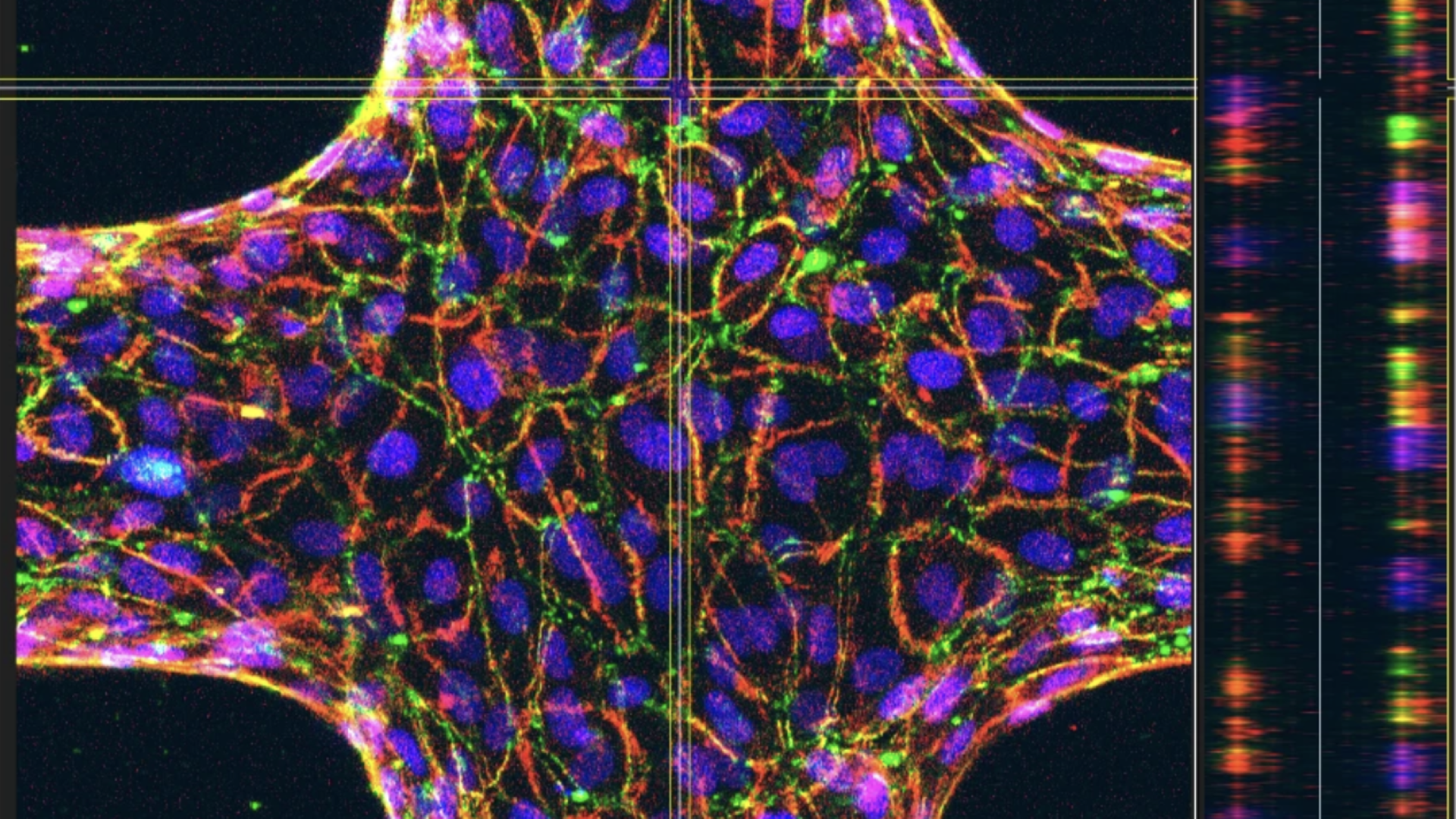
Researchers from the Liverpool School of Tropical Medicine, Universidade Católica Portuguesa, Gulbenkian Institute for Molecular Medicine Lisbon, and EMBL Barcelona developed the first physiologically accurate 3D models of bovine brain and heart microvessels to study how T. congolense parasites attach to blood vessel walls, a process called sequestration, which helps the parasite evade clearance.
The team discovered key molecular mechanisms for sequestration and demonstrated that the process is influenced by blood flow and regulated by a signalling pathway that helps the parasite grow. These insights could be used to develop new treatments aimed at disrupting this process. The study also challenges previous ideas by showing that sequestration is not necessary for the parasite to be transmitted to tsetse flies, which then spread it to other cows.
Traditional lab models are limited to experiments in culture flasks and on flat, plastic surfaces. The new methods used in this study create a 3D structure from blood vessel cells designed to mimic a real bovine blood vessel. This lets researchers study how the parasites interact with blood vessels in a much more realistic way.
Dr Aitor Casas-Sanchez said: “These findings bring us closer to understanding why T. congolense infections lead to severe or fatal disease.
“The methods used to make this discovery are revolutionary. By mimicking bovine blood vessels in vitro, the natural environment of the parasite, we’ve been able to dissect the biological and biophysical factors that underpin sequestration, something that was previously impossible with traditional models.”
Animal African trypanosomiases are a major burden on livestock health across sub-Saharan Africa, leading to millions of dollars in economic losses every year.
In severe cases, especially when the brain is affected, large numbers of parasites can build up in a cow’s blood vessels, leading to stroke-like symptoms and death. Understanding the role of sequestration in disease progression could open new avenues for targeted interventions.
Dr Casas-Sanchez added: “This study not only sheds light on the fundamental biology of T. congolense but also provides a platform to test potential sequestration inhibitors. Similar bioengineered models have transformed malaria research, and we anticipate they will have a similar impact on trypanosomiasis.
“This is a significant leap forward in our understanding of trypanosome biology. It underscores the power of bioengineering tools in tackling complex infectious diseases and brings us closer to strategies that could alleviate the burden of these diseases on affected communities.”
The research also suggests that sequestration may offer a metabolic advantage to the parasite, supporting its spread throughout the body. Future work will explore the molecular interactions between the parasite and host cells, with the goal of identifying druggable targets to disrupt this process.
By refining the 3D models used in this study and expanding their use, researchers aim to uncover new strategies to mitigate the impact of T. congolense on livestock.