STRESST
Antimicrobial Stewardship in Hospitals, Resistance Selection and Transfer in a One Health Context

The STRESST project (Antimicrobial Stewardship in Hospitals, Resistance Selection and Transfer in a One Health Context) will determine the effects of hospital-wide antimicrobial stewardship on the amount of antibiotics, and the numbers of susceptible and resistant bacteria, released in hospital wastewater.
Hospital wastewater is likely to represent a hotspot for selection of antibiotic resistant bacteria therefore the project will show if stewardship can reduce the amounts of antibiotics and resistant bacteria entering the environment.
Ultimately, the STRESST project will demonstrate the effectiveness of antimicrobial stewardship in reducing antibiotic, and antibiotic resistant bacteria, release into the environment and how this impacts resistance transmission within and between microbial communities present in animals that use this as a water source. This holistic view of resistance transmission within a One Health context will serve to highlight a selection hotspot (hospital wastewater) for future interventions.
STRESST is funded via the JPIAMR
STRESST will determine if the antibiotics present can catalyse both intracellular transposition and intercellular conjugation of mobile genetic elements carrying antibiotic resistance genes, and if this is less likely to occur following the antimicrobial stewardship intervention.
In addition to agar plate-based assays, these experiments will also include state of the art animal caecum fermenter models which will directly link the healthcare environment (hospital) with the wider environment (via hospital wastewater) and animal health representing all three One Health areas.

The workplan
Antimicrobial Stewardship
Antimicrobial Stewardship interventions will be introduced across Queen Elizabeth Central Hospital in Blantyre, Malawi.

Hospital wastewater acquisition
Bespoke hospital wastewater sewage samplers will be used to acquire samples before and during the hospital wide antimicrobial stewardship interventions.
Microbiological analysis of hospital wastewater
Susceptible and resistant bacteria will be grown, identified and enumerated. The mechanisms of resistance will be determined.

Chemical analysis of hospital wastewater
The hospital wastewater will be analysed by chemists to determine the levels of antibiotics that are leaving the hospital ground in the hospital wastewater.

Analysis of horizontal gene transfer of antimicrobial resistance genes
Rates of transfer by conjugation will be determined in the presence of different concentrations of antimicrobials found in the hospital wastewater by using state of the art fermenters which allow us to model the chicken caecum.
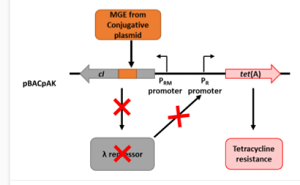
Analysis of intracellular transposition of antimicrobial resistance genes
The ability of mobile genetic elements to jump between plasmids or between plasmids and the chromosome inside a bacterial cell will be determined using newly developed molecular biology assays.
Supporting studies
Pedersen, Torunn & Tellevik, Marit & Kommedal, Oyvind & Lindemann, Paul & Moyo, Sabrina & Janice, Jessin & Blomberg, Bjørn & Samuelsen, Ørjan & Langeland, Nina. (2020). Horizontal Plasmid Transfer among Klebsiella pneumoniae Isolates Is the Key Factor for Dissemination of Extended-Spectrum β-Lactamases among Children in Tanzania. mSphere. 5. 10.1128/mSphere.00428-20.
, , , , , . Patterns of community assembly in the developing chicken microbiome reveal rapid primary succession. MicrobiologyOpen. 2019; 8:e821. https://doi.org/10.1002/mbo3.821
Ingrid Cardenas-Rey, Teresita Bello Gonzalez, Jeanet Van der Goot et al. Succession in the Caecal Microbiota of Developing Broilers Colonised by Extended-spectrum β-lactamase-producing Escherichia Coli, 27 September 2021, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-929974/v1]
Lester R, Haigh K, Wood A, MacPherson EE, Maheswaran H, Bogue P, Hanger S, Kalizang'oma A, Srirathan V, Kulapani D, Mallewa J, Nyirenda M, Jewell CP, Heyderman R, Gordon M, Lalloo DG, Tolhurst R, Feasey NA. Sustained Reduction in Third-generation Cephalosporin Usage in Adult Inpatients Following Introduction of an Antimicrobial Stewardship Program in a Large, Urban Hospital in Malawi. Clin Infect Dis. 2020 Dec 3;71(9):e478-e486. doi: 10.1093/cid/ciaa162. PMID: 32060523; PMCID: PMC7713689.
Tansirichaiya S, Moyo SJ, Al-Haroni M, Roberts AP. Capture of a novel, antibiotic resistance encoding, mobile genetic element from Escherichia coli using a new entrapment vector. J Appl Microbiol. 2021 Mar;130(3):832-842. doi: 10.1111/jam.14837. Epub 2020 Sep 17. PMID: 32881179.
Tansirichaiya S, Goodman RN, Guo X, Bulgasim I, Samuelsen Ø, Al-Haroni M, Roberts AP. Intracellular Transposition and Capture of Mobile Genetic Elements following Intercellular Conjugation of Multidrug Resistance Conjugative Plasmids from Clinical Enterobacteriaceae Isolates. Microbiol Spectr. 2022 Jan 19;10(1):e0214021. doi: 10.1128/spectrum.02140-21. Epub ahead of print. PMID: 35044219; PMCID: PMC8768599.
Principal Investigators
|
Dr Adam Roberts |

Dr Andrew Singer |

Prof Nina Langeland |

Dr Michael Brouwer |
Professor Nicholas Feasey |
Project Manager

Rocio Villacorta Linaza
Project Manager







